Mass Spectra Interpretation: ALDEHYDE
Quiz Instructions:
Electron impact Mass Spectrometry produced the spectra below. Many Peaks are labelled to aid interpretation: M/Z (relative abundance). Look at the Hints for help. You will need a Periodic Table, a Table of Common Isotopes, a calculator, and scratch paper to work the quiz.
No. of Questions = 8
1. How many oxygens are in the molecular ion peak in this spectrum?
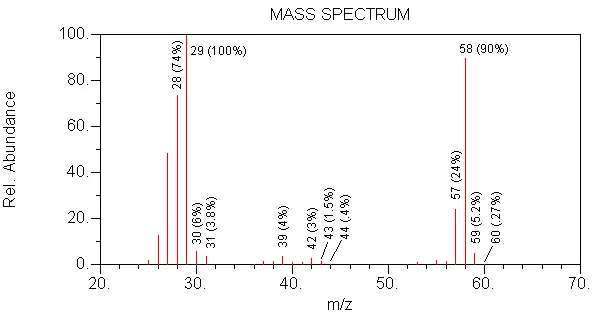
WRONG --> (0.27/90)x100=0.3, 0.3/0.2=1 oxygen
CORRECT --> (0.27/90)x100=0.3, 0.3/0.2=1 oxygen
WRONG --> (0.27/90)x100=0.3, 0.3/0.2=1 oxygen
2. For this same spectrum, choose the compound that the spectrum represents.

WRONG --> MW is too high (72)
CORRECT --> MW is 58, 29 is formed by loss of 29 (CH2CH3) from 58
WRONG --> MW is too low (56)
WRONG --> this compound is not an aldehyde
WRONG --> in this compound, 2 oxygens would cause the m/z 60 isotope peak to be 0.40 relative to m/z 58
3.Find the alkyl ion series in the spectra below. (Check the hint!)
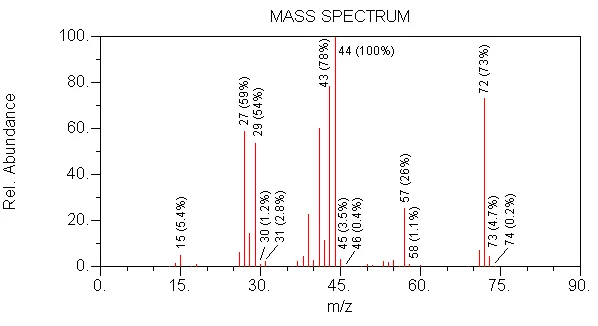
CORRECT --> CH3, CH2CH3, CH2CH2CH3
WRONG --> this is the alkyl ion loss series
WRONG --> there is a series
4.Find the alkyl LOSS ion series in the same spectra shown below. (Check the hint!)

WRONG --> this is the alkyl series
CORRECT --> this is the loss series observed in the spectra: 72-43=29, 72-29=43, 72-15=57
WRONG --> there is a series
5.For this same spectrum, choose the compound that the spectrum represents.

WRONG --> MW is too low (56)
WRONG --> in this compound, branching would reduce the intensity of 57
WRONG --> MW is too high (86)
WRONG --> in this compound, 2 oxygens would cause the m/z 74 isotope peak to be 0.40 relative to m/z 72
CORRECT --> MW is 72, 44 is formed by loss of 28 (C2H4) from 72
6. Choose the compound that this spectrum represents.
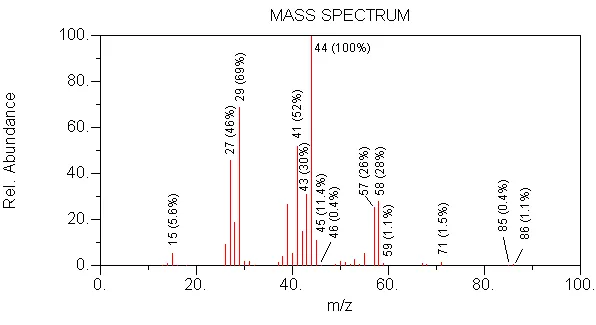
WRONG --> MW is too high (100)
WRONG --> in this compound, branching would increase the intensity of the molecular ion
CORRECT --> MW is 86, 44 is formed by loss of 28 (C2H4) from 86
WRONG --> MW is too low (72)
WRONG --> in this compound, stability of a quaternary carbon would produce an intense 57
7. Choose the compound that this spectrum represents.
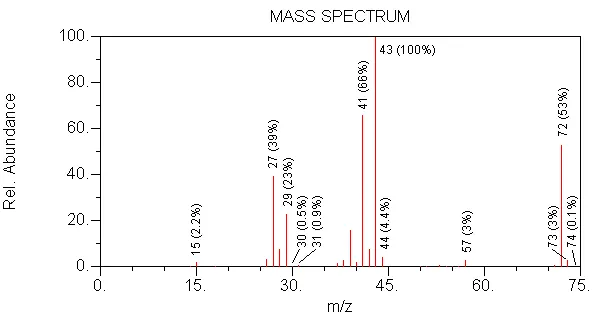
CORRECT --> MW is 72, 43 is formed by loss of 28 (C2H4) from 72
WRONG --> in this compound, no branching would increase the intensity of 57
WRONG --> in this compound, 2 oxygens would cause the m/z 74 isotope peak to be 0.40 relative to m/z 72
WRONG --> MW is too low (56)
WRONG --> MW is too high (86)
8. Choose the compound that this spectrum represents.
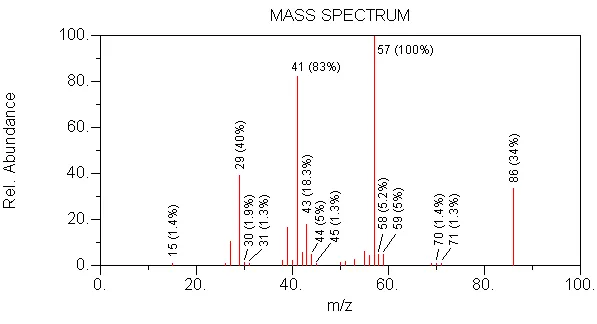
CORRECT --> MW is 86, 57 is formed by loss of 29 (HCO) from 86
WRONG --> MW is too high (100)
WRONG --> MW is too low (72)
WRONG --> in this compound, the secondary carbon would increase the intensity of 43
WRONG --> in this compound, no branching would reduce 57 and the molecular ion would be less intense






