
The Department of Chemistry & Biochemistry is committed to providing students with engaging learning experiences both inside and outside of the classroom. Our accredited online courses are professionally designed by experienced web developers provided by the Office of the Provost and monitored on a regular basis for quality and content by the UA Office of Digital Learning and Student Engagement. Additional online courses will be coming soon.
Register for all online courses through UA Online. Register for BIOC Online courses.
CHEM 130 - Chemistry for Allied & Public Health
CHEM 130 is a one-semester lecture course, designed to introduce students to the fundamentals of chemistry as a foundation of many central topics in allied health fields. It provides an overview of the principles of general and organic chemistry and elements of biochemistry, emphasizing medical, nutritional, and environmental aspects of the discipline. Current topics in health sciences are used to guide students in developing a solid background in chemistry that may be applied in their future careers.
The topics chosen for coverage in this online course were selected to best serve the interests and needs of undergraduate students preparing for careers in Allied Health, such as pre-Nursing, Speech Language and Hearing, and Public Health students. Students majoring in chemistry, biochemistry, chemical engineering, or materials science may wish to enroll in Chemistry 151 or 181, which are courses designed with these majors in mind. Please consult with your advisor.
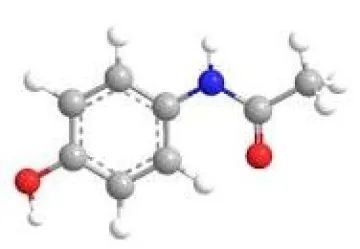
Structure of Acetaminophen (Tylenol)
Course Objectives
The objective of CHEM 130 is to introduce the students to the fundamentals of general, organic and biochemistry, focusing on the applications to nursing and public health. Critical thinking and pattern recognition are utilized with the goal of developing skills in problem solving, applying the foundations of chemistry to new concepts. Students are taught to integrate their conceptual and modeling skills with quantitative data to make predictions regarding the behavior of molecules in different environments. The course adopts a flexible pedagogical approach allowing students to combine instructor-guided and self-paced study formats, depending on their individual circumstances, needs, and learning styles. The following skills will be developed:
- Use correct dosing and the metric system in areas such as medicine, units in environmental chemistry, safety, and toxicity.
- Use trends in periodic properties to predict chemical and physical properties of the elements. Construct a Lewis structure for a covalent compound, including molecules with expanded and incomplete octet configurations.
- Recognize chemical building blocks, how structure relates to function, chemical & physical properties, reactivity recognition, and hydrogen bonding in biological systems.
- Understand how substances interact and why, such as predicting whether a reaction will proceed, IV medication safety, pH & pharmaceutical design, and physiological processes.
- Carry out calculations involving limiting reactants, with a focus on green chemistry and reducing hazardous & chemical waste.
- Understand how the basic material and trends in the periodic table are relevant to new applications in organic reactions, naming, and identification.
- Have a working knowledge of organic molecules and functional groups, as well as discuss their role in chemical reactions and physical interactions.
- Demonstrate understanding of key concepts in biochemistry including structure-function relationships in biologically important macromolecules, and the chemical basis of food metabolism including understanding the organic reactions and conditions involved.
Prerequisites
This course is designed for non-technical students with minimum science or math backgrounds. Algebra is recommended.
Required Textbook
This course uses the VitalSource/McGraw Hill/Pearson/Mastering Chemistry software package associated with the source text, “General, Organic, and Biological Chemistry: Structures of Life” 6thedition by Karen Timberlake, Pearson (2019)
If you register for the course, you should have access to this software package through Inclusive Access. Also required are a desktop or laptop computer, a stable broadband wired or wireless internet connection, a webcam, speakers or headphones, and a microphone.
Example Syllabus: CHEM 130 Spring 2024 Syllabus
Example Course Schedule: Spring 2024 Course Schedule
CHEM 141 – General Chemistry Lecture I: Quantitative
CHEM 141 is the first part of a two-semester lecture series introducing students to the central principles of modern chemistry using a quantitative atoms-first approach. The course is intended for students who require a strong foundation in general chemistry, rooted in a technical (mathematical) approach to the discipline. It specifically targets science and engineering majors and other students interested in a systematic development of modern chemistry.
Course Objectives
- Have a basic understanding of the physical principles defining the structures and fundamental properties of atoms, molecules, and the states of matter;
- Have a working knowledge of the periodic trends and be able to use the Periodic Table to describe the properties of atoms;
- Understand the basic quantum-mechanical concepts involved in chemical bonding and the fundamental principles defining molecular geometries;
- Be proficient in the quantitative description of chemical reactions and stoichiometry;
- Be able to apply mathematical techniques and the laws of Physics to solve quantitative chemical problems, including the application of critical thinking, metric system, conversion of units, and scientific notation;
- Be able to integrate the conceptual understanding and quantitative problem solving skills to describe, analyze, and model the structure and common properties of matter.
Recommended Prerequisites
Appropriate math placement level OR Proctored/Prep for College Algebra 88+ OR Proctored/Prep for Calculus 65+ OR MATH 109C, 110, 112, 113, 120, 120R, 124, 125, 129, 223.
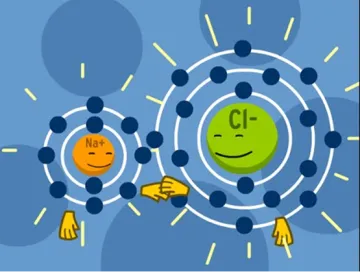
Sodium Chloride
Required Textbook
Brown, LeMay, Bursten, Chemistry: The Central Science, 14th ed. (required). Inclusive Access online ebk and homework platform.
Example Syllabus: CHEM 141 Fall 2025 Syllabus
Example Course Schedule: Fall 2025 Course Schedule
CHEM 142 – General Chemistry Lecture II: Quantitative
HEM 142 is the second part of a two-semester lecture series introducing students to the central principles of modern chemistry using a quantitative approach. It focuses on the principles of thermodynamics, chemical equilibrium, and chemical kinetics. No special mathematical background is needed, beyond the stated prerequisite requirements. The course aims to develop a deep understanding of general chemistry, rooted in a formal approach to the discipline, and is intended for students who wish to apply this knowledge to real problems they will encounter in their future careers. The course is designed for all science and engineering majors, pre-medical and pre-pharmacy students, and is appropriate for any beginning student.
Course Objectives
- Understand the basic principles of Thermodynamics as they apply to chemical reaction including changes in internal energy, enthalpy, entropy, and free energy
- Predict the properties of solutions and determine how enthalpy and entropy changes along with the physical environment such as temperature will determine solubility
- Understand chemical equilibrium and be able to predict concentrations of reactants and products at equilibrium
- Study various types of equilibria in detail such as acid/base reactions, and aqueous equilibria
- Determine how Gibbs Free energy controls equilibrium and how temperature effects it.
- Understand the basics of electrochemistry and be able to predict what species is oxidized and what reduced. Calculate standard cell potentials.
- Understand the basics of chemical kinetics and rate laws and use this information to predict plausible chemical mechanisms of reactions.
- Understand the basics of nuclear chemistry and both fission and fusion.
Recommended Prerequisites
Chem 141. Appropriate math placement level OR Proctored/Prep for College Algebra 88+ OR Proctored/Prep for Calculus 65+ OR MATH 109C, 110, 112, 113, 120, 120R, 124, 125, 129, 223.
Required Textbook
Brown, LeMay, Bursten, Chemistry: The Central Science, 14th ed. (required). Inclusive Access online ebk and homework platform.
Example Syllabus: CHEM 142 Summer 2025 Syllabus
Example Course Schedule: CHEM 142 Summer 2025 Schedule
The first semester of a two-semester laboratory sequence designed to provide an introduction to the central principles and practices of modern quantitative chemical analysis.
Example Syllabus: CHEM 145 Fall 2025 Syllabus
Example Course Schedule: Fall 2025 Course Schedule
The second semester of a two-semester laboratory sequence designed to provide an introduction to the central principles and practices of modern quantitative chemical analysis.
Example Syllabus: CHEM 146 Summer 2025 Syllabus
Example Course Schedule: Summer 2025 Course Schedule
General principles of organic chemistry.
Example Syllabus: CHEM 241A Fall 2025 Syllabus
CHEM 241B – Lectures in Organic Chemistry
(Second Semester)
The topics chosen for coverage in this online course were selected to best serve the interests and needs of undergraduate students majoring in any of the Life Sciences, as well as other pre-professional health sciences students.
Students majoring in chemistry, biochemistry, chemical engineering, or materials science may wish to enroll in Chemistry 246B, which is a course designed with these these majors in mind. Please consult with your advisor.
Structure of Oseltamivir (Tamiflu)
Course Objectives
The Chemistry 241A/241B lecture series teaches the fundamentals of organic chemistry with a focus on organic structures, properties, transformations (organic synthesis), and the mechanisms of those transformations (organic reaction mechanisms). The second semester course, CHEM 241B, covers the chemistry of radicals, the chemistry of dienes, the chemistry of carbonyl-containing functional groups (including aldehydes, ketones, carboxylic acids, acid chlorides, acid anhydrides, esters, and amides), the chemistry of amines (including imines, enamines, and nitriles), the chemistry of carbohydrates (including a brief introduction to nucleic acids), the chemistry of amino acids (including a brief introduction to peptides and proteins), and the chemistry of lipids.
Prerequisites
Chemistry 241A, 242A, 246A, or an equivalent first-semester organic chemistry lecture course. If your grade in the first semester organic chemistry lecture was below a “C”, you are strongly urged to retake that course before proceeding on to the second semester organic chemistry lecture course.
Required Textbook
This course uses the Connect/LearnSmart/SmartBook/Proctorio software package associated with the source text, "Organic Chemistry", Sixth Edition, by Janice Gorzynski Smith. If you register for the course, you should have access to this software package through Inclusive Access. Also required are a desktop or laptop computer, a stable broadband wired or wireless internet connection, a webcam, speakers or headphones, and a microphone.
Example Syllabus: CHEM 241B Summer 2025 Syllabus
BIOC 384 - Foundations in Biochemistry
Fundamental concepts in physical biochemistry (energy conversion, water, and membranes), protein structure/function, methods in protein biochemistry, enzyme mechanisms, protein-mediated cell signaling, and fundamental energy conversion pathways.
This course is designed for undergraduate students with majors in any of the Life Sciences and other pre-professional health science students. Bioc 384 is the companion course to Bioc 385 “Metabolic Biochemistry.” Note that it is possible to take them out of sequence since Bioc 384 is not the prerequisite for Bioc 385.
Course Objectives
- Demonstrate proficiency with vocabulary used in biochemistry.
- Describe energy conversion processes and the role of membranes in biochemistry.
- Describe the structure of nucleic acids and core methods in nucleic acid and protein biochemistry.
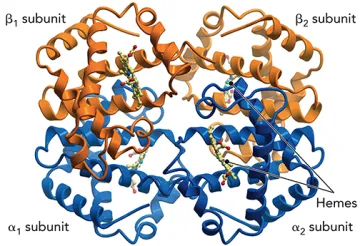
- Explain the principles of oxygen transport and protein transport across biological membranes.
- Describe the catalytic properties, kinetics, and allosteric regulation of metabolic enzymes.
- Understand the key differences between four major signal transduction pathways in eukaryotes.
- Describe the enzymatic reactions and bioenergetics of glucose metabolism.
Recommended Prerequisites
MCB 181R (Biology)
CHEM 152 (General Chemistry)
CHEM 241A (Organic Chemistry)
A prerequisite waiver can be an option
Required Textbook
Miesfeld & McEvoy Biochemistry, available at the UA bookstore and online.
Example Syllabus: BIOC 384 Fall 2025 Syllabus
Example Course Schedule: Fall 2025 Course Schedule
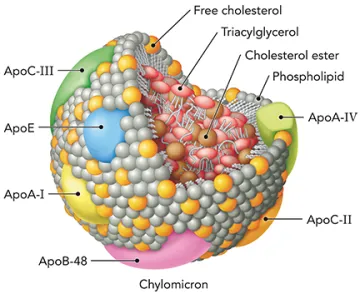
BIOC 385 - Metabolic Biochemistry
Fundamentals of metabolism at the cellular and organismal levels, with a focus on regulatory mechanisms that control metabolic flux. Topics include metabolic flux through energy converting pathways, metabolism of carbohydrates, lipids, amino acids, and nucleotides.
This course is designed for undergraduate students with majors in any of the Life Sciences and other pre-professional health science students. Bioc 385 is the companion course to Bioc 384 “Foundations in Biochemistry.” Note that it is possible to take them out of sequence since Bioc 384 is not the prerequisite for Bioc 385.
Course Objectives
- Describe the meaning of metabolic flux and mechanisms of metabolic regulation.
- Describe the relationship between molecular structure and function of biomolecules.
- Describe the enzymatic reactions and regulation of key carbohydrate metabolic pathways.
- Describe the enzymatic reactions and regulation of key lipid metabolic pathways.
- Describe the enzymatic reactions and regulation of key amino acid metabolic pathways.
- Describe the enzymatic reactions and regulation of key nucleotide metabolic pathways.
- Describe the key biochemical processes mediating DNA, RNA, and protein metabolism.
Recommended Prerequisites
MCB 181R (Biology)
CHEM 152 (General Chemistry)
CHEM 241A (Organic Chemistry)
A prerequisite waiver can be an option
Required Textbook
Miesfeld & McEvoy Biochemistry, available at the UA bookstore and online.
Example Syllabus: BIOC 385 Fall 2025 Syllabus
Example Course Schedule: Summer 2025 Course Schedule






