Distinguished Professor
Degrees and Appointments
- BS, MS 1973-79, San Diego State University
- PhD 1979-83, Stony Brook University
- Fellow 1983-87, UC San Francisco
Field of Study: Biochemistry
Awards and Honors
- University Distinguished Professor, 2018
- Tech Launch Arizona Campus Collaborator of the Year, 2018
- UA Honors College Faculty Excellence Award, 2012
- Chair, American Cancer Society TBE Panel, 1999-2001
- Chair, Gordon Conference on Cancer, 1994
- Fellow & Scholar, Leukemia Society America, 1986-1994
- Fellow, Jane Coffin Childs Memorial Fund, 1983-1986
Research Specialties: Chemical Biology, Chemistry & Biochemistry Education, Metabolism, Signaling, and Regulation, Nucleic Acids and Genomes, Protein and Membrane Biochemistry
Research
Mosquitoes are human disease vectors that transmit pathogens through blood feeding. These human pathogens include the malarial parasite, Dengue, Zika, and yellow fever viruses, and West Nile virus. Dengue fever and malaria continue to have a significant health and economic impact worldwide, and therefore new approaches are needed for controlling the spread of these diseases. Mosquitoes are highly evolved blood sucking insects that have been shown to efficiently ingest, metabolize, and transport blood meal nutrients from the midgut lumen to the ovaries to produce eggs. Although we have a basic knowledge of these processes at the physiological level, it requires a molecular understanding of metabolic regulation in mosquitoes in order to target blood meal metabolism as transmission blocking or vector control strategy. Since highly coordinated endocytotic and exocytotic processes in midgut, fat body, and ovary tissues likely play a central role in metabolic flux following the blood meal, we are investigating vesicle transport in Aedes aegypti and Anopheles stephensi mosquitoes.
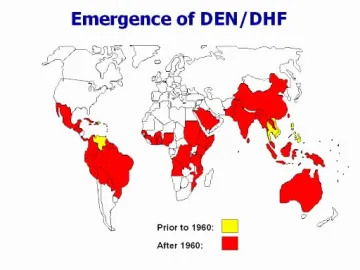
Increase in reported cases of Dengue hemorrhagic fever (WHO)
OVERVIEW OF BLOOD MEAL METABOLISM IN MOSQUITOES
Newly emerged female mosquitoes feed on nectar for several days until they are able to take their first blood meal (males do not blood feed). The blood meal is required for Ae. aegypti egg development and results in the deposition of ~100 fertilized eggs within ~60 hours of feeding. In order to produce this many eggs, blood meal metabolism requires efficient retrieval of nutrients and rapid excretion of toxic ammonia. This is an amazing accomplishment considering the mosquito's size. A typical female Ae. aegypti female mosquito weighs ~2.5 mg and can consume a blood meal of 2 ul in ~60 seconds. This 2.5 mg meal (including the water, protein, and lipid) is therefore equal in mass to her own body. This would be equivalent to a 125 lb. women drinking a 12 gallon smoothie that contains 25 lbs. of hamburger meat, 0.5 lb. of butter, and 2 tbls of sugar. Can you imagine not only drinking this mega smoothie in less than a minute, but completely digesting it, and then excreting all of the toxic waste products in just 24 hours? The female Ae. aegypti mosquito does this up to five times in her lifetime, resulting in the production of 500+ mosquito eggs over a two week period.

REGULATION OF EGGSHELL SYNTHESIS IN FEMALE MOSQUITOES
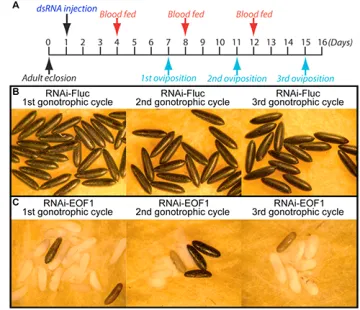
Mosquito-borne diseases are responsible for several million human deaths annually around the world. One approach is to disrupt molecular processes or antagonize novel metabolic targets required for the production of viable eggs as a means of controlling mosquito populations. To this end, we focused our efforts on identifying proteins required for completion of embryonic development that are mosquito-selective and represent potential targets for vector control. We performed bioinformatic analyses to identify putative protein-coding sequences that are specific to mosquito genomes. Systematic RNAi screening of 40 mosquito-specific genes was performed by injecting dsRNA into female Aedes aegypti mosquitoes. This experimental approach led to the identification of eggshell organizing factor 1 (EOF-1), which plays an essential role in the formation and melanization of the eggshell. Eggs deposited by EOF1-deficient mosquitoes have non-melanized fragile eggshells, and all embryos are non-viable. Scanning electron microscopy analysis identified that exochorionic eggshell structures are strongly affected in EOF1-deficient mosquitoes. EOF1 is a potential novel target for exploring the identification and development of mosquito-selective and biosafe small molecule inhibitors.
We examined the effect of EOF1 deficiency on eggs in three consecutive gonotrophic cycles in individual containers. Eggshell melanization, fecundity, and viability phenotypes are profoundly altered in EOF1-deficient mosquitoes during the first three gonotrophic cycles. Therefore, our data demonstrate that the RNAi-EOF1 effect from a single dsRNA injection remains substantial for the second and even the third gonotrophic cycles.
MOLECULAR ANALYSIS OF LIPID METABOLISM IN MOSQUITOES
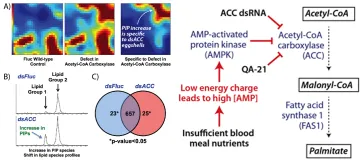
To better understand the mechanism of de novo lipid biosynthesis in blood fed Ae. aegypti mosquitoes, we quantitated acetyl-CoA carboxylase (ACC) and fatty acid synthase 1 (FAS1) transcript levels in blood fed mosquitoes, and used RNAi methods to generate ACC and FAS1 deficient mosquitoes. Using the ketogenic amino acid 14C-leucine as a metabolic precursor of 14C-acetyl-CoA, we found that 14C-triacylglycerol and 14C-phospholipid levels were significantly reduced in both ACC and FAS1 deficient mosquitoes, confirming that ACC and FAS1 are required for de novo lipid biosynthesis after blood feeding. Surprisingly however, we also found that ACC deficient mosquitoes, but not FAS1 deficient mosquitoes, produced defective oocytes, which lacked an intact eggshell and gave rise to inviable eggs. This severe phenotype was restricted to the 1st gonotrophic cycle, suggesting that the eggshell defect was due to ACC deficiencies in the follicular epithelial cells, which are replaced after each gonotrophic cycle. Consistent with lower amounts of de novo lipid biosynthesis, both ACC and FAS1 deficient mosquitoes produced significantly fewer eggs than control mosquitoes in both the 1st and 2nd gonotrophic cycles. Lastly, FAS1 deficient mosquitoes, but not ACC deficient mosquitoes, showed delayed blood meal digestion, suggesting that a feedback control mechanism may coordinate rates of fat body lipid biosynthesis and midgut digestion during feeding. We propose that decreased ACC and FAS1 enzyme levels lead to reduced 14C-lipid biosynthesis and lower fecundity, whereas altered levels of the regulatory metabolites acetyl-CoA and malonyl-CoA account for the observed defects in eggshell formation and blood meal digestion, respectively.
Publications listed in Google Scholar
Jun Isoe , Carter J. Simington, Max E. Oscherwitz, Alyssa J. Peterson, Alberto A. Rascon Jr., Brooke B. Massani, Roger L. Miesfeld and Michael A. Riehle (2023) Characterization of essential eggshell proteins from Aedes aegypti mosquitoes., BMC Biology 21:214.
Miesfeld, R.L. and McEvoy, M.M. (2021) BIOCHEMISTRY Textbook, Second Edition, WW Norton Publishing, New York, NY, 1392 pages.
Isoe, J., Koch, L.E., Isoe, Y.E., Rascon, A.A, Brown, H.E., Massani, B.B., and Miesfeld, R.L. (2019) Identification and characterization of a mosquito-specific eggshell organizing factor in Aedes aegypti mosquitoes, PLOS Biology, January 8, PMID 30620728.
Isoe, J., Stover, W., Miesfeld, R.B. and Miesfeld, R.L. (2013) COPI-mediated blood meal digestion in vector mosquitoes is independent of midgut ARF-GEF and ARF-GAP regulatory activities., Insect. Bioc. Mol. Biol., 43:732-739.
Mack, D.J., Isoe, J., Miesfeld, R.L. and Njardarson, J.T. (2012) Distinct biological effects of Golgicide A derivatives on larval and adult mosquitoes, Bioorg. Med. Chem. Lett., 22:5177-5188.
Alabaster, A., Isoe, J., Zhou, G., Lee, A., Murphy, A. and Miesfeld, R. (2011) Deficiencies in acetyl-CoA carboxylase and fatty acid synthase 1 differentially affect eggshell development and blood meal digestion in Aedes aegypti. Insect. Bioc. Mol. Biol., 41:946-955.
Rascon, A., Gearin, J., Isoe, and Miesfeld, R. (2011) In vitro activation and enzyme kinetic analysis of recombinant midgut serine proteases in the Dengue vector mosquito Aedes aegypti. BMC Bioc. 12:43.
Isoe, J., Collins, J., Badgandi, H., Day, W.A., and Miesfeld, R. (2011) Defects in COPI transport cause blood feeding-induced mortality in Yellow Fever mosquitoes, Proc. Nat. Acad. Sci., 108:E211-E217.
Zhou, G., Isoe, J., Day, W.A., and Miesfeld, R. (2011) Alpha-COPI transport function is required for rough endoplasmic reticulum whorl formation in mosquito midgut epithelial cells, PLoS ONE 6(3): e18150.
Scaraffia, P.Y., Zhang, Q., Thorson, K., Wysocki, V.H. and Miesfeld, R. (2010) Differential ammonia metabolism Aedes aegypti fat body and midgut tissues, J. Insect. Physiol., 56:1040-1049.
Wei, J., Wysocki, V.H., Miesfeld, R. and Scaraffia, P.Y. (2010) Differentiation and quantification of C1 and C2 13C-labeled glucose by tandem mass spectrometry, Analytical. Bioc., 404:40-44.
Brackney, D.E., Isoe, J., Zamora, J., Black, W.C., Foy, B.D., Miesfeld, R. and Olson, K.E. (2010) Expression profiling and comparative analysis of seven novel midgut serine proteases from the Yellow Fever mosquito, J. Insect. Physiol. 56:736-744.
Isoe, J., Rascon, A., Kunz, S., and Miesfeld, R. (2009) Molecular genetic analysis of midgut serine proteases in Aedes aegypti mosquitoes, Insect Bioc. Mol. Biol., 39:903-912.
Zhou, G. and Miesfeld, R. (2009) Differential utilization of blood meal amino acids in Aedes aegypti mosquitoes, Open Access Insect Physiology, 1:1-12.
Zhou, G. and Miesfeld, R. (2009) Energy metabolism during diapause in Culex pipiens mosquitoes. J. Insect. Physiol., 55:40-46.
Isoe, J., Zamora, J. and Miesfeld, R. (2009) Molecular analysis of the Aedes aegypti carboxypeptidase gene family. Insect. Bioc. Mol. Biol., 39:68-73.
Scaraffia, P.Y., Tang, G., Isoe, J., Wysocki, V.H., Wells, M.A. and Miesfeld, R. (2008) Discovery of an alternate metabolic pathway for urea synthesis in adult Aedes aegypti mosquitoes, Proc. Nat. Acad. Sci., 105:518-523.
Brandon, M.C., Pennington, J.E., Zamora, J., Isoe, J., Schillinger, A-S. and Miesfeld, R. (2008) TOR signaling is required for amino acid stimulation of early trypsin protein synthesis in the midgut of Aedes aegypti mosquitoes. Insect. Bioc. Mol. Biol., 38:916-922.
Isoe, J., Kunz, S., Manhart, C., Wells, M.A. and Miesfeld, R. (2007) Regulated expression of microinjected DNA in adult Aedes aegypti mosquitoes. Insect Mol. Biol., 16:83-92.
mosquitovector biologyDengueegg developmenteverydaybiochemistry






