Associate Professor
Degrees and Appointments
- B.S. Chemistry, 1989, Northern Arizona University
- Ph.D. Chemistry, 1996, University of California, Santa Barbara
- Postdoctoral Fellow, 1996-1998, Department of Cell Biology and Physiology, University of New Mexico School of Medicine
- Cancer Research Fellow, 1998-2002, National Cancer Institute, NIH
Field of Study: Biochemistry, Inorganic Chemistry
Awards and Honors
- NSF CAREER Award, 2007
- Presidential Early Career Award for Scientists and Engineers (PECASE), 2008
- Achievement Award for Outstanding Mentor of Graduate/Professional Students (GPS), GPS Council, 2010
- Fellow of the American Association for the Advancement of Science (AAAS), 2013
- College of Science Distinguished Advising Award, University of Arizona, 2014
Research Specialties: Bioanalytical, Bioinorganic, Chemical Biology, Metabolism, Signaling, and Regulation, Protein and Membrane Biochemistry, Spectroscopy/Molecular Structure, Synthesis/Synthetic Methods Development
Research
Cell survival, development, replication, repair and adaptation depend on the ability to sense and respond to acute and chronic environmental changes. One mechanism by which cells detect, communicate and adjust to changing conditions involves chemical modifications to proteins, DNA and lipids. A subset of these modifications is mediated by small, redox active molecules, including reactive oxygen species (ROS), which are produced by reduction of molecular oxygen, and reactive nitrogen species (RNS), which typically originate from and are more oxidized than nitric oxide (NO). Redox signaling is a major component of intracellular communication, immune response and wound healing but can also contribute to chemical stress and cell death when dysregulated.
Understanding the chemical and biological basis for the differences in outcome of redox signaling agent production is a challenge that my research program is addressing, with the goal of identifying critical factors and markers of disease development. We also produce nitrogen oxide donors as analytical and pharmacological tools, with a long-term goal of harnessing signaling mechanisms and or modulating key targets for the treatment of cancer, heart failure, pain and alcoholism.
Such an undertaking requires a multidisciplinary, collaborative approach that involves synthesis of new chemical donors, development of analytical techniques to detect nitrogen oxides and modified biomolecules, and analysis of pharmacological effects within cultured cells and in animal models.
Synthesis and characterization of chemical donors of nitrogen oxides
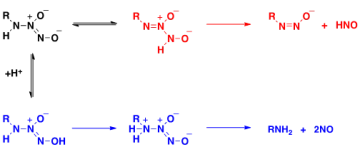
Donor systems offer many experimental advantages including ease and cost of use, safe handling and controllable rates of release of reactive species. However, it can be difficult to clearly identify the products of donor decomposition, especially in cellular systems. One component of research in the Miranda lab is to produce new donor systems, particularly of NO and HNO, and to characterize these compounds both structurally and mechanistically. An example is our determination that one class of donor can release either HNO or NO depending on pH. This type of characterization assists in both experimental design and interpretation of data.
Detection
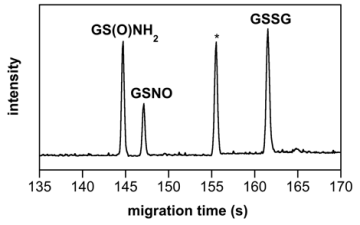
Many redox active signaling agents are highly reactive, which limits lifetime and complicates detection. We seek to design detection systems that are selective for a specific signaling agent, that have sufficient sensitivity for use in cells or tissue and that can provide both quantitative and real-time data. Since redox signaling inherently meets these criteria, we have utilized a natural target – thiols or -SH containing-compounds – as the basis for a detection system. We are now able to separate and quantify various nitrogen oxide-induced modifications of glutathione (the major intracellular thiol; GSH) with ultra-high resolution and with sufficient sensitivity to detect in relatively small numbers of cells. We are currently utilizing this method to verify production of redox active signaling agents by cells and by chemical donors within cells.
Drug development
Modifications to our nitrogen oxide donors can tune properties such as solubility and decomposition rate and can introduce new functionalities that are useful in terms of drug design. One such route is to attach chemical moieties that enhance drug delivery or targeting. Another is to couple our donors with existing drugs to produce an additive or synergistic effect. We are currently developing conjugates of our donors with nonsteroidal anti-inflammatory drugs (NSAIDs) such as aspirin as anticancer agents.
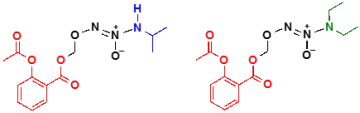
Adducts of this type retain the anti-inflammatory properties of the NSAID as well as the nitrogen oxide donor properties (HNO = blue; IPA/NO-aspirin or NO = green; DEA/NO-aspirin). In addition, these conjugates are significantly more effective in reducing cancer cell growth compared to the parent compounds both in culture and in animal models.
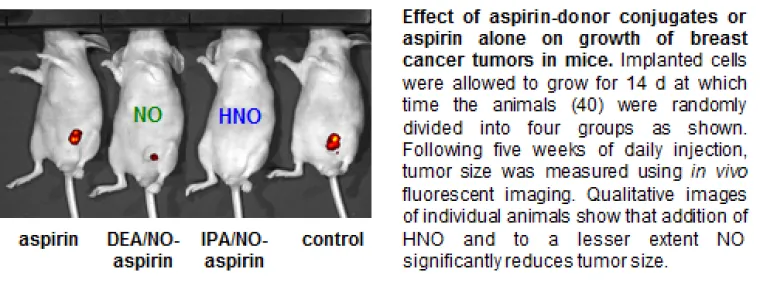
These conjugates are also not appreciably cytotoxic toward non-tumorigenic cells, suggesting a cancer-specific sensitivity that is promising for chemotherapy or chemoprevention.
Target analysis
Given the high reactivity of redox active signaling agents, redox signaling depends on fine control, meaning precise modulation of a few targets among many. Correlating chemical modifications to biological outcomes can contribute to understanding of disease development and can establish targets for drug therapy. To this end, we are currently engaged in the investigation of the reactivity of nitrogen oxides at the molecular, biomolecular, systemic and whole organism levels. For example, fundamental kinetic analysis suggested to us that thiols were major targets for HNO. This led us to measure the effects of our HNO-donating aspirin adduct on the expression and/or activity of several proteins both known to contribute to tumor growth and to contain a cysteine residue. Such data can then be related to for example reduced metabolism, which may in part be responsible for the reduced tumor size shown above. We are now progressing to more comprehensive analysis of the cellular effects of redox active signaling agent production via gene expression, genomic and proteomic techniques.
D.D. Thomas, J.L. Heinecke, L.A. Ridnour, R. Cheng, A.H. Kesarwala, C.H. Switzer, D.D. Roberts, S. Glynn, J.M. Fukuto, D.A. Wink*, K.M. Miranda*, "Nitrosative Signaling and Stress: the Redox Landscape in NOS2 biology", Free Radic. Biol. Med., 87, 204-225 (2015).
D. Basudhar, R.C. Cheng, G. Bharadwaj, L.A. Ridnour, D.A. Wink, K.M. Miranda*, "Chemotherapeutic potential of diazeniumdiolate-based aspirin prodrugs in breast cancer", Free Radic. Biol. Med., 83, 101-114 (2015).
J.H. Jorolan, L.A. Buttitta, C. Cheah, K.M. Miranda*, "Comparison of the chemical reactivity of synthetic peroxynitrite with that of the autoxidation products of nitroxyl or its anion", Nitric Oxide, 44, 39-46 (2015).
R.Y.S. Cheng, D. Basudhar, L.A. Ridnour, J. Heinecke, A.H. Kesarwala, S. Glynn, C.H. Switzer, S. Ambs, K.M. Miranda, D.A. Wink*, "Gene expression profiles of NO- and HNO-donor treated breast cancer cells: Insights into tumor response and resistance pathways", Nitric Oxide, 43, 17-28 (2014).
G.M. Johnson, T.J. Chozinski, E.S. Gallagher, C.A. Aspinwall, K.M. Miranda*, "Glutathione sulfinamide serves as a selective, endogenous biomarker for nitroxyl following exposure to therapeutic levels of donors", Free Radic. Biol. Med., 76, 299-307 (2014).
L. Staurengo-Ferrari, A.C. Zarpelon, D.T. Longhi-Balbinot, M. Marchesi, T.M. Cunha, J.C. Alves-Filho, F.Q. Cunha, S.H. Ferreira, R. Casagrande, K.M. Miranda, W.A. Verri, Jr*, "Nitroxyl inhibits overt pain-like behavior in mice: role of cGMP/PKG/ATP-sensitive potassium channel signaling pathway", Pharmacol. Rep., 66, 691-698 (2014).
D. Basudhar, G. Bharadwaj, R.Y. Cheng, S. Jain, S. Shi, L.A. Ridnour, V.M. Caceres, R.C. Spadari-Bratfisch, N. Paolocci, C.A. Velázquez-Martínez, D.A. Wink, K.M. Miranda*, "Synthesis and comparison of nitroxyl and nitric oxide releasing diazeniumdiolate-based aspirin derivatives", J. Med. Chem., 56, 7804-7820 (2013).
T. Heinrich, R. da Silva, K.M. Miranda, C.H. Switzer, D.A. Wink, J.M. Fukuto*, "Biological nitric oxide signaling: chemistry and terminology", Br. J. Pharmacol., 169, 1417-1429 (2013).
G.M. Johnson, T.J. Chozinski, D.J. Salmon, A.D. Moghaddam, H.C. Chen. K.M. Miranda*, "Quantitative detection of nitroxyl upon trapping with glutathione and labeling with a specific fluorogenic reagent", Free Radic. Biol. Med., 63, 476-484 (2013).
Corrigendum, Free Radic. Biol. Med., 77, 390 (2014).
D.J. Salmon, C.L. Torres de Holding, L. Thomas, K.V. Peterson, G.P. Goodman, J.E. Saavedra, A.Srinivansan, K.M. Davies, L.K. Keefer, K.M. Miranda*, "HNO and NO release from a primary amine-based diazeniumdiolate as a function of pH", Inorg. Chem., 50, 3262-3270 (2011).
D. Andrei, D.J. Salmon, S. Donzelli, A. Wahab, J.R. Klose, M.L. Citro, J.E. Saavedra, D.A. Wink, K. M. Miranda*, Larry K. Keefer*, "Dual mechanisms of HNO generation by a nitroxyl prodrug of the diazeniumdiolate (NONOate) class", J. Am. Chem. Soc., 132, 16526-16532 (2010).
T.W. Miller, M.E. Cherney, N. Franco, P.J. Farmer, S.B. King, A.J. Hobbs, K.M. Miranda, J.N. Burstyn, J.M. Fukuto*, "The effects of nitroxyl (HNO) on soluble guanylate cyclase activity: interactions at ferrous heme and cysteine thiols", J. Biol. Chem, 284, 21788-21796 (2009).
S. Donzelli, M.G. Espey, W. Flores-Santana, C.H. Switzer, G.C. Yeh, J. Huang, D. Stuehr, S.B. King, K. M. Miranda, D.A. Wink*, "Generation of nitroxyl by heme protein-mediated peroxidation of hydroxylamine but not N-hydroxy-L-arginine", Free Radic. Biol. Med., 45, 578-584 (2008).
A.S. Dutton, C.P. Suhrada, K.M. Miranda, D.A. Wink, J.M. Fukuto, K.N. Houk*, "Mechanism of pH dependent decomposition of monoalkylamine diazeniumdiolates to form HNO and NO, deduced from Density Functional Theory and CBS-QB3 calculations", Inorg. Chem., 45, 2448-2456 (2006).
K.M. Miranda*, T. Katori, C.L. Torres de Holding, L. Thomas, L.A. Ridnour, W.J. McLendon, S. Cologna, A.S. Dutton, H.C. Champion, D. Mancardi, C.G. Tocchetti, J. Saavedra, L.K. Keefer, K.N. Houk, J.M. Fukuto, D.A. Kass, N. Paolocci, D.A. Wink*, "Comparison of the NO and HNO donating properties of diazeniumdiaolates. Primary amine adducts release HNO in vivo", J. Med. Chem., 48, 8220-8228 (2005).
K.M. Miranda*, H.T. Nagasawa, J.P. Toscano, "Donors of HNO", Curr. Topics Med. Chem., 5, 649-664 (2005).
K.M. Miranda*, A.S. Dutton, L.A. Ridnour, C.A. Foreman, E. Ford, N. Paolocci, T. Katori, D. Mancardi, D.D. Thomas, M.G. Espey, K.N. Houk, J.M. Fukuto, D.A. Wink*, "Mechanism of aerobic decomposition of Angeli’s salt (sodium trioxodinitrate) at physiological pH", J. Am. Chem. Soc., 127, 722-731 (2005).
K.M. Miranda, "The chemistry of nitroxyl (HNO) and implications in biology", Coordin. Chem. Rev., 249, 433-455 (2005).
K.M. Miranda*, N. Paolocci, T. Katori, D.D. Thomas, M.D. Bartberger, M.G. Espey, D.A. Kass, M. Feelisch, J.M. Fukuto, D.A. Wink*, "A biochemical rationale for the orthogonal behavior of nitroxyl and nitric oxide in the cardiovascular system", Proc. Natl. Acad. Sci. USA, 100, 9196-9201 (2003).
N. Paolocci, T. Katori, H.C. Champion, M.E. St. John, K.M. Miranda, J.M. Fukuto, D.A. Wink, D.A. Kass*, "Positive inotropic and lusitropic effects of HNO/NO- in failing hearts: independence from β-adrenergic signaling", Proc. Natl. Acad. Sci. USA, 100, 5537-5542 (2003).
M.D. Bartberger, W. Liu, E. Ford, K.M. Miranda, C. Switzer, J.M. Fukuto*, P.J. Farmer, D.A. Wink, K.N. Houk*, "The reduction potential of nitric oxide (NO) and its importance to NO biochemistry", Proc. Natl. Acad. Sci. USA, 99, 10958-10963 (2002).
K.M. Miranda*, M.G. Espey, D.A. Wink, "A rapid, simple spectrophotometric method for detection of nitrate and nitrite", Nitric Oxide, 5, 62-71 (2001).
K.M. Miranda*, M.G. Espey, K. Yamada, M. Krishna, N. Ludwick, S. Kim, D. Jourd’heuil, M.B. Grisham, M. Feelisch, J.M. Fukuto, D.A. Wink, "Unique oxidative mechanisms for the reactive nitrogen oxide species, nitroxyl anion", J. Biol. Chem., 276, 1720-1727 (2001).






