University Distinguished Professor
Degrees and Appointments
- Professor 2016 - Present, University of Arizona, Department of Chemistry and Biochemistry
- Associate Professor 2010-2016, University of Arizona, Department of Chemistry and Biochemistry
- Assistant Professor 2004-2010, Cornell University, Department of Chemistry and Chemical Biology
- Assistant Professor 2005-2010, Tri-Institutional: Rockefeller University, MSKCC and Weill-Cornell
- Postdoctoral Fellow 2001-2004, Memorial Sloan-Kettering Cancer Center (with Professor Samuel J. Danishefsky)
- Ph.D. 1994-2001, Yale University
- B.S. 1990-1993, University of Iceland, Reykjavik, Iceland
Field of Study: Organic Chemistry
Awards and Honors
- Japan Society for the Promotion of Science (JSPS) Fellowship, 2018
- University of Arizona Distinguished Scholar Award, 2016
- Merrill Presidential Scholar Outstanding Educator, 2009
- Thieme Chemistry Journal Award, 2008
- Marilyn Emmons Williams Award, 2006
- General Motors Cancer Research Scholar Program Award, 2001-2003
- Helgu Jonsdottir and Sigurlida Kristjansson Memorial Foundation Award, 1995
Research Specialties: Synthesis/Synthetic Methods Development
Research
The main objectives of our research program at the University of Arizona are twofold: 1) Development and study of useful new synthetic strategies and methods for organic chemistry. 2) Total synthesis of complex natural products exhibiting unique biological activity. In our laboratory, these two research areas are usually closely linked. All of our synthetic blueprints are expected to showcase a new synthetic method or a unique disconnection that is ideally suited for the target architecture thus ensuring a short and efficient synthesis, which in turn provides us with access to valuable intermediates and products for deciphering molecular mechanisms and biological evaluation. By adhering to such stringent design criteria, we find without exception that a fertile environment for new ideas is invariably created. The harmony between new reaction development and natural product total synthesis serves also to showcase the strengths of the new method over existing methods while at the same time enabling access to the natural product. It is important also not to forget that in addition to the valuable training that total synthesis provides students, the unanticipated challenges and the realization of limitations of existing methods that most good synthetic plans are faced with constantly remind us that the richest source of new ideas is often the synthetic journey itself.
The need for operationally simple atom-efficient methods with broad substrate scope is great. Practical new synthetic methods not surprisingly are of high value to society since a large number of research areas rely on building new molecular architecture either for fundamental research or industrial applications such as pharmaceuticals, commodity chemicals and polymers. My research group is dedicated to the development of useful new synthetic methods (Figure 1). Over the last few years we have initiated research programs focused on the development of 1) new catalytic ring expansions of strained heterocycles, 2) new useful anionic cascade reactions and 3) oxidative deromatization strategies and reactions.
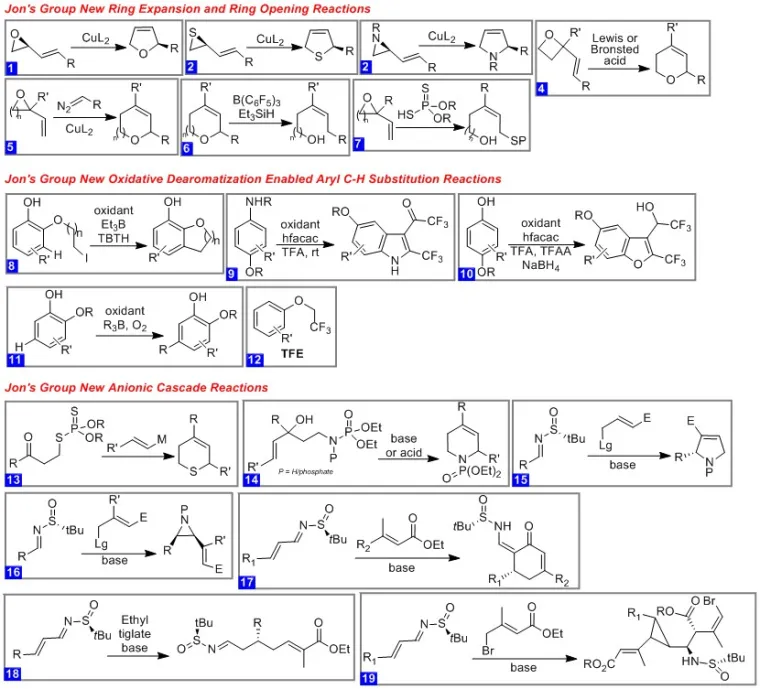
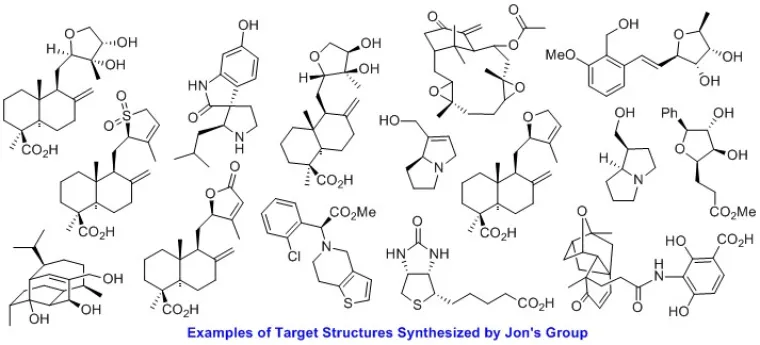
Our complex molecule synthetic efforts are primarily focused on the total synthesis of unique bicyclic diterpenoid natural products with promising anti-cancer and antibiotic functions. Our concise synthetic approaches demonstrate the usefulness of a class of oxidative dearomatization reactions that serve as the key step in many of these total syntheses. The efficient routes to these complex and structurally diverse set of bicyclic natural products under investigation in our group serve as a testament to the power of such dearomatization focused retrosynthetic strategies. Our long-term goal is the development of useful asymmetric variants of these oxidative transformations. Given the rigid architecture of these targets and how sparingly they are functionalized, we expect that a relatively small collection of hybrid structures obtained by diverting the synthetic blueprint will prove sufficient to learn more about their function. This knowledge in combination with our flexible synthetic blueprint will allow us to design new and improved natural product-derived anticancer and antibiotic agents.
Our group collaborates extensively with other research groups. We currently have active collaborations in drug discovery (Page, Peti, Zarnescu, Polt and Chapman groups), inorganic/organometallic chemistry (Lichtenberger group) & analytical chemistry (Aspinwall and Heien group).
We are committed to developing new and creative web-accessible organic chemistry teaching tools. Towards that end we created the TOP 200 DRUG POSTERS, DISEASE FOCUSED POSTERS, STRUCTURE POSTERS and CHEMISTRY BY DESIGN, which is a new educational website and application (APP) focused on the synthesis of natural products and pharmaceuticals. Links to these can be found on our group homepage.
62) “Efforts Toward a Unified Kainoid Family Synthesis Approach: Unexpected Sulfinamide Directed Conjugate Addition Results” Chogii, I.; Das, P.; Njardarson, J. T. Asian. J. Org. Chem. 2019, 8, ASAP.
61) "A Survey of the Structures of US FDA Approved Combinatino Drugs" Das, P.; Delost, M. D.; Qureshi, M.; Smith, D. T.; Njardarson, J. T. J. Med. Chem. 2019, 62, 4265.
60) "Asymmetric Vinylkogous aza-Darzens Approach to Vinyl Aziridines" Chogii, I.; Das, P.; Delost, M. D.; Njardarson, J. T. Org. Lett. 2018, 20, 4942.
59) "From Oxiranes to Oligomers: Architectures of US FDA Approved Pharmaceuticals Containing Oxygen Heterocycles" Delost, M. D.; Smith, D. T.; Anderson, B. J.; Njardarson, J. T. J. Med. Chem. 2018, 61, 10996.
58) "Review of Synthetic Approaches Toward Maoecrystal V" Smith, B. R.; Njardarson, J. T. Org. Biomol. Chem. 2018, 16, 4210.
57) "[2.2.2] to [3.2.1]-Bicycle Skeletal Rearrangement Approach to the Gibberellin Family of Natural Products" Smith, B. R.; Njardarson, J. T. Org. Lett. 2018, 20, 2993.
56) "Analysis of US FDA Drugs Containing Sulfur Atoms" Scott, K. A.; Njardarson, J. T. Topics in Current Chemistry - Sulfur Chemistry. 2018, 376, 1.
55) "Double-Diels-Alder Approach to Maoecrystal V. Unexpected C-C Bond-Forming Fragmentations of the [2.2.2]-Bicyclic Core" Smith, B. R.; Njardarson, J. T. Org. Lett. 2017, 19, 5316.
53) "New Class of Anioin-Accelerated Amino-Cope Rearrangements as Gateway to Diverse Chiral Structures" Chogii, I.; Das, P.; Fell, J. S.; Scott, K. A; Crawford, M. N.; Houk, K. N.; Njardarson, J. T. J. Am. Chem. Soc. 2017, 139, 13141.
52) "Dearomatization Approach to 2-Trifluoromethylated Benzofuran and Dihydrobenzofuran Products" Smith, D. T.; Vitaku, E.; Njardarson, J. T. Org. Lett. 2017, 19, 3508.
51) "A Mild Meta-Selective C-H Alkylation of Catechol Mono-Ethers" Vitaku, E.; Njardarson, J. T. Eur. J. Org. Chem. 2016, 3679.
50) "Anionic Cascade Routes to Sulfur and Nitrogen Heterocycles Originating from Thio- and Aminophosphate Precursors" Das, P.; Njardarson, J. T. Eur. J. Org. Chem. 2016, 4249.
49) "Metal-Free Synthesis of Fluorinated Indoles Enabled by Oxidative Dearomatization" Vitaku, E.; Smith, D. T.; Njardarson, J. T. Angew. Chem. Int. Ed. 2016, 55, 2243.
48) "Asymmetric [3+2] Annulation Approach to 3-Pyrrolines: Concise Total Syntheses of (-)-Supinidine, (-)-Isoretronecanol and (+)-Elacomine" Chogii, I.; Njardarson, J. T. Angew. Chem. Int. Ed. 2015, 54, 13706.
47) “Synthesis of 1,2,3,6-Tetrahydropyridines via Aminophosphate Enabled Anionic Cascade and Acid Catalyzed Cyclization Approaches” Das, P.; Njardarson, J. T. Org. Lett. 2015, 17, 4030.
46) "Synthetic Approaches and Total Syntheses of Vinigrol, a Unique Diterpenoid" Draghici, C.; Njardarson, J. T. Tetrahedron 2015, 71, 3775.
45) "Ring Expansions of Oxiranes and Oxetanes" Smith, D. T.; Njardarson, J. T. Topics in Heterocyclic Chemistry 2015, 41, 281(book chapter).
44) “The Realization of an Oxidative Dearomatization-Intramolecular Diels-Alder Route to Vinigrol”, Draghici, C.; Njardarson, J. T. Strategies and Tactics in Organic Synthesis 11 (Book chapter) 2015, accepted.
43) "Formation of Fused Aromatic Architectures via an Oxidative Dearomatization - Radical Cyclization Rearomatization Approach" Vitaku, E.; Njardarson, J. T. Tetrahedron Lett. 2015, 56, 3550 (Special issue in honor of Professor H. H. Wasserman)
42) "Analysis of the Structural Diversity, Substitution Patterns and Frequency of Nitrogen Heterocycles among US FDA Approved Pharmaceuticals" Vitaku, E.; Smith, D. T.; Njardarson, J. T. J. Med. Chem. 2014, 57, 10257.
41) "Beyond C, H, O and N! Analysis of the Elemental Composition of US FDA Approved Drug Architectures" Smith, B. R.; Eastman, C. M.; Njardarson, J. T. J. Med. Chem. 2014, 57, 9764.
40) "Mild Stereoselective Formation of Tri- and Tetrasubstituted Olefins by Regioselective Ring Opening of 1,1-Disubstituted Vinyl Oxiranes with Dialkyl Dithiophosphates" Guo, B.; Vitaku, E.; Njardarson, J. T.; Li, F.; Smith, B. R.; Das, P. Tetrahedron Lett. 2014, 55, 3232.
39) "Evolution of an Oxidative Dearomatization Enabled Total Synthesis of Vinigrol" Yang, Q.; Draghici, C.; Njardarson, J. T.; Li, F.; Smith, B. R.; Das, P. Org. Biomol. Chem. 2014, 12, 330.
38) "A Scalable Rhodium-Catalyzed Intermolecular Aziridination" Smith, D. T.; Njardarson, J. T. Angew. Chem. Int. Ed. 2014, 53, 4278.
37) "Data-mining for Sulfur and Fluorine: An Evaluation of Pharmaceuticals To Reveal Opportunities for Drug Design and Discovery" Ilardi, E. A.; Vitaku, E.; Njardarson, J. T. J. Med. Chem. 2014, 57, 2832.
36) "Total Synthesis of Vinigrol" Yang, Q.; Njardarson, J. T.; Draghici, C. D.; Li, F. Angew. Chem. Int. Ed. 2013, 52, 8648.
35) "Z-Selective Ring Opening of Vinyl Oxetanes with Dialkyl Dithiophosphate Nucleophiles" Yang, Q.; Njardarson, J. T.; Draghici, C. D.; Li, F. Chem. Commun. 2013, 49, 10802.
34) "Syntheses and Structural Confirmations of Members of a Heterocycle-Containing Family of Labdane Diterpenoids" Mack, D. J.; Njardarson, J. T. Angew. Chem. Int. Ed. 2013, 52, 1543.
33) "Ring Expansions of Vinyl- Oxiranes, Thiiranes and Aziridines: Synthetic Approaches, Challenges and Catalytic Success Stories" Ilardi, E. A.; Njardarson, J. T. J. Org. Chem. 2013, 78, 9533.
32) "Mechanism and the Origins of Stereospecificity in Copper Catalyzed Ring Expansion of Vinyl Oxiranes: A Traceless Dual Transition Metal-Mediated Process" Mustard, T. J. L.; Mack, D. J.; Njardarson, J. T.; Cheong, Paul. H.-Y. J. Am. Chem. Soc. 2013, 134, 1471.
31) "An In-Pharm-ative Educational Poster Anthology Highlighting the Therapeutic Agents that Chronicle Our Medicinal History" Ilardi, E. A.; Vitaku, E.; Njardarson, J. T. J. Chem. Ed. 2013, 90, 1403.
30) "Base Mediated Deprotection Strategies for Trifluoroethyl (TFE) Ethers, a New Alcohol Protecting Group" Yang, Q.; Njardarson, J. T. Tetrahedron Lett. 2013, 54, 7080.
29) "Recent Advances in the Metal-Catalyzed Ring Expansions of Three- and Four-Membered Rings" Mack, D. J.; Njardarson, J. T. ACS Catal. 2013, 3, 272.
28) "Catalytic Ring Expansion Adventures" Njardarson, J. T. Synlett 2013, 787.
27) "Reactions at your fingertips" Njardarson, J. T. Nature Chem. 2012, 4, 519.
26) "New Mechanistic Insights into the Copper Catalyzed Ring Expansion of Vinyl Aziridines: Evidence in Support of a Copper(I) Mediated Pathway" Mack, D. J.; Njardarson, J. T. Chem. Sci. 2012, 3, 3321.
25) "Synthesis of Allylic and Homoallylic Alcohols from Unsaturated Cyclic Ethers Using a Mild and Selective C-O Reduction Approach" Mack, D. J.; Guo, B.; Njardarson, J. T. Chem. Commun. 2012, 48, 7844.
24) "Catalytic Ring Expansion of Vinyl Oxetanes. Asymmetric Synthesis of Chiral Dihydropyrans Using Chiral Counterion Catalysis" Guo, B.; Schwarzwalder, G.; Njardarson, J. T. Angew. Chem. Int. Ed. 2012, 51, 5675.
23) "Efficient Synthesis of Thiopyrans Using Sulfur-Enabled Anionic Cascade" Li, F.; Calabrese, D.; Brichacek, M.; Lin, Y.; Njardarson, J. T. Angew. Chem. Int. Ed. 2012, 51, 1938.
22) "Chemistry By Design - A Web Based Educational Flashcard for Exploring Synthetic Organic Chemistry" Draghici, C.; Njardarson, J. T. J. Chem. Ed. 2012, 89, 1080.
21) "Distinct Biological Effects of Golgicide A Derivatives on Larval and Adult Mosquitos" Mack, D. J.; Isoe, J.; Miesfeld, R. L.; Njardarson, J. T. Bioorg. Med. Chem. Lett. 2012, 22, 5177.
20) "Pharmaceutical Structure Montages as Catalysts for Design and Discovery" Njardarson, J. T. Future Med. Chem. 2012, 4, 951.
19) "Intermolecular Oxonium Ylide Mediated Synthesis of Medium-Sized Oxacycles" Mack, D. J.; Batory, L. A.; Njardarson, J. T. Org. Lett. 2012, 14, 378.
18) "Synthetic Efforts Toward [3.3.1] Bridged Bicyclic Phloroglucinol Natural Products" Njardarson, J. T. Tetrahedron 2011, 67, 7631.
17) "Emergence of Potent Inhibitors of Metastasis in Lung Cancer vis Syntheses Based on Migrastatin" Lecomte, N.; Njardarson, J. T.; Nagorny, P.; Yang, G.; Downey, R.; Ouerfelli, O.; Moore, M. A. S.; Danishefsky, S. J. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 15074.
16) "Stereospecific Ring Expansion of Chiral Vinyl Aziridines" Brichacek, M.; Navarro-Villalobos, M.; Plichta, A.; Njardarson, J. T. Org. Lett. 2011, 13, 1110.
15) " An Efficient Oxidative Dearomatization-Radical Cyclization Approach to Symmetrically Substituted Bicyclic Guttiferone Natural Products" McGrath, N. A.; Binner, J. R.; Markopoulos, G.; Brichacek, M.; Njardarson J. T. Chem. Commun. 2011, 47, 209.
14) "Stereoselective Ring Expansion of Vinyl Oxiranes. Mechanistic Insights and Natural Product Total Synthesis" Brichacek, M.; Batory, L. A.; Njardarson, J. T. Angew. Chem. Int. Ed. 2010, 49, 1648.
13) "The Strategic Marriage of Method and Motif. Total Synthesis of Varitriol" Brichacek, M.; Batory, L. A.; McGrath, N. A.; Njardarson J. T. Tetrahedron 2010, 66, 4832.
12) "A Graphical Journey of Innovative Organic Architectures That Have Improved Our Lives" McGrath, N. A.; Brichacek, M.; Njardarson J. T. J. Chem. Ed. 2010, 87, 1348.
11) "A Concise Ring Expansion Route to the Compact Core of Platensimycin" McGrath, N. A.; Bartlett, E. S.; Sittihan, S.; Njardarson, J. T. Angew. Chem., Int. Ed. 2009, 48, 8543.
10) "Rapid Assembly of Vinigrol's Carbocyclic Core" Morton, J. G. M.; Dragichi, C.; Kwon, L. D.; Njardarson, J. T. Org. Lett. 2009, 11, 4492.
9) "Creative Approaches Towards the Synthesis of 2,5-Dihydro- Furans, Thiophenes and Pyrroles. One Method Does Not Fit All!" Brichacek, M.; Njardarson, J. T. Org. Biomol. Chem. 2009, 7, 1761.
8) "Efforts Towards the Total Synthesis of Vinigrol" Morton, J. G. M.; Kwon, L. D.; Freeman, J. D.; Njardarson, J. T. Synlett 2009, 23.
7) "An Adler-Becker Oxidation Approach to Vinigrol" Morton, J. G. M.; Kwon, L. D.; Freeman, J. D.; Njardarson, J. T. Tetrahedron Lett. 2009, 50, 1684.
6) "Lewis Acid Catalyzed [1,3]-Sigmatropic Rearrangement of Vinyl Aziridines" Brichacek, M.; Njardarson, J. T.; Lee-D.-L. Org. Lett. 2008, 10, 5023.
5) "An Efficient Substrate Controlled Synthesis of Hypoestoxide, a Member of a Unique Family of Diterpenoid Natural Products with an Inside-Out [9.3.1]Bicyclic Core" McGrath, N. A.; Lee, C. A.; Araki, H.; Brichacek, M.; Njardarson, J. T. Angew. Chem. Int. Ed. 2008, 47, 9450.
4) "PET Imaging of Soluble Yttrium-86-Labeled Carbon Nanotubes in Mice" McDewitt, M. R.; Chattopadhyay, D.; Jaggi, J. S.; Finn, R. D.; Zanzonico, P. B.; Villa, C.; Rey, D.; Mendenhall, J.; Batt, C. A.; Njardarson, J. T.; Scheinberg, D. A. PLos One 2007, 2.
3) "Tumor Targeting with Antibody-Functionalized Radiolabled Carbon Nanotubes" McDewitt, M. R.; Chattopadhyay, D.; Kappel, B. J.; Jaggi, J. S.; Schiffman, S. R.; Antczak, C.; Njardarson, J. T.; Brentjens, R.; Scheinberg, D. A. J. Nuc. Med. 2007, 48, 1180.
2) "Highly Selective Copper-Catalyzed Ring Expansion of Vinyl Thiiranes: Application to Synthesis of Biotin and the Heterocyclic Core of Plavix®" Rogers, E.; Araki, H.; Batory, L. A.; McInnis, C. E.; Njardarson, J. T. J. Am. Chem. Soc. 2007, 129, 2768.
1) "Copper Catalyzed Rearrangement of Vinyl Oxiranes" Batory, L. A.; McInnis, C. E.; Njardarson, J. T. J. Am. Chem. Soc. 2006, 128, 16054.






