Professor
Degrees and Appointments
- B.S. 1975, The University of California, Irvine
- B.A. 1975, The University of California, Irvine
- Ph.D. 1980, The University of Utah
- NIH Postdoctoral Fellow 1981-1983, Harvard University
Field of Study: Organic Chemistry
Awards and Honors
- UA CoS Distinguished Career Teaching Award, 2009
- UA CoS Innovation in Teaching Award, 1995
- IBM Paul J Flory Fellow, 1990-1991
- National Institutes of Health Postdoctoral Fellow, 1981-1983
Research Specialties: Bioorganic, Materials and Polymer Chemistry, Synthesis/Synthetic Methods Development
1. Imaging and Drug Delivery to the Gastrointestinal Tract
We have developed untargeted molecules such as 1 based on a sucrose scaffold for use in magnetic resonance imaging of and drug delivery to the gastrointestinal tract (see Figure 1). We are now working to develop targeted versions of these molecules for early detection and treatment of diseased tissues.
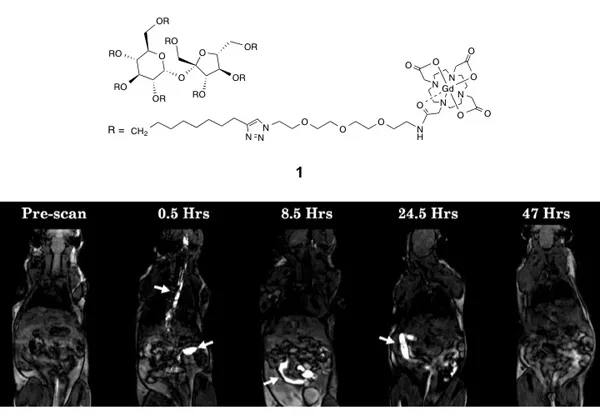
Figure 1. T1-weighted GE3D images of the Gd-DOTA/sucrose construct 1 passing through the GI tract of a C3H mouse. Arrows indicate points of bright CA-related contrast. At 0.5 h post-gavage, bright contrast is observed in the esophagus and stomach; at 8.5 h contrast is observed in the small intestine; at 24.5 h contrast is observed in the large intestine; and by 47 h all contrast has cleared the GI tract.
2. Polymers Containing Large Bicyclic Rings
Elasticity of polymeric materials is generally attributed to chain entanglement. Materials with untangled, aligned chains make good fibers, but often have limited elasticity. This research seeks to prepare polymers that can pack as untangled, aligned chains, but that do possess significant elasticity due to the conformational properties of one or both co-monomers incorporated during polymerization (see Figure 2).
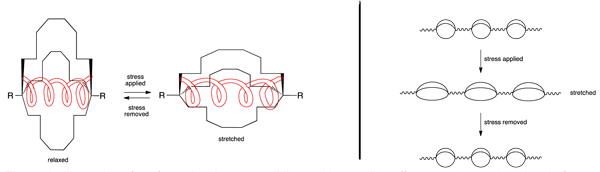
Figure 2. Illustration of conformational compressibility and its possible effect on polymer elasticity. Left: 1,10-Disubstituted bicyclo[8.8.8]hexacosane as a molecular spring. Right: Stress response of polymers incorporating large bicyclo[m.m.m]alkane ring systems.
Mason, D. J.; Timofeyenko, Y. G.; Jagadish, B.; Mash, E. A. Synth. Commun. 2022, 52, 1825-1833. "Palladium-catalyzed Hydrogenations in Dichloromethane."
Menezes, O.; Kadoya, W. M.; Gavazza, S.; Sierra-Alvarez, R.; Mash, E. A.; Abrell, L. M.; Field, J. A. J. Hazardous Mater. 2021, 413, 125459. "Covalent Binding with Model Quinone Compounds Unveils the Environmental Fate of the Insensitive Munitions Reduced Product 2,4-Diaminoanisole (DAAN) under Anoxic Conditions."
Kadoya, W. M.; Sierra-Alvarez, R.; Jagadish, B.; Wong#, S.; Abrell, L. M.; Mash, E. A.; Field, J. A. Environmental Pollution 2021, 268B, 115682. "Covalent Bonding of Aromatic Amine Daughter Products of 2,4-Dinitroanisole (DNAN) with Model Quinone Compounds Representing Humus via Nucleophilic Addition."
Kadoya, W. M.; Sierra-Alvarez, R.; Jagadish, B.; Wong#, S.; Abrell, L. M.; Mash, E. A.; Field, J. A. Chemosphere 2019, 222, 789-796. "Coupling Reactions between Reduced Intermediates of Insensitive Munitions Compound 4-Nitroanisole."
Kadoya, W. M.; Sierra-Alvarez, R.; Wong#, S.; Abrell, L. M.; Mash, E. A.; Field, J. A. Chemosphere 2018, 195, 372-380. "Evidence of Anarobic Coupling Reactions between Reduced Intermediates of 4-Nitroanisole."
Jagadish, B.; Hurley, N. R.; Mash, E. A. Synth. Commun. 2017, 47, 2395-2398. "Solvent-free Nitration of Electron-rich Arenes."
Raghunand, N.; Scicinski, J.; Guntle, G. P.; Jagadish, B.; Mash, E. A.; Bruckheimer, E.; Oronsky, B.; Korn, R. L. Oncotarget 2017, 8, 102511-102520. "Magnetic Resonance Imaging of RRx-001 Pharmacodynamics in Preclinical Tumors."
Landowski, T. H.; Guntle, G. P.; Zhao, D.; Jagadish, B.; Mash, E. A.; Dorr, R. T.; Raghunand, N. Translational Oncology 2016, 9, 228-235. "Magnetic Resonance Imaging Identifies Differential Response to Pro-Oxidant Chemotherapy in a Xenograft Model."
Dehigaspitiya, D. C.; Anglin, B. L.; Smith, K. R.; Weber, C. A.; Lynch, R. M.; Mash, E. A. Org. Biomol. Chem. 2015, 13, 11507-11517. "Linear Scaffolds for Multivalent Targeting of Melanocortin Receptors."
Elshan, N. G. R. D.; Jayasundera, T.; Weber, C. A.; Lynch, R. M.; Mash, E. A. Bioorg. Med. Chem. 2015, 23, 1841-1848. "Development of a Time-resolved Fluorescence Probe for Evaluation of Competitive Binding to the Cholecystokinin 2 Receptor."
Dehigaspitiya, D. C.; Navath, S.; Weber, C. A.; Lynch, R. M.; Mash, E. A. Tetrahedron. Lett. 2015, 56, 3060-3065. "Synthesis and Bioactivity of MSH4 Oligomers Prepared by an A2 + B2 Strategy."
Elshan, N. G. R. D.; Jayasundera, T.; Anglin, B. L.; Weber, C. A.; Lynch, R. M.; Mash, E. A. Org. Biomol. Chem. 2015, 13, 1778-1791. "Trigonal Scaffolds for Multivalent Targeting of Melanocortin Receptors."
Elshan, N. G. R. D.; Patek, R.; Vagner, J.; Mash, E. A. Anal. Biochem. 2014, 464, 24-29. "Spectrophotometric Determination and Removal of Unchelated Europium Ions from Solutions Containing Eu-Diethylenetriaminepentaacetic Acid Chelate-Peptide Conjugates."
Jagadish, B.; Ozumerzifon, T. J.; Roberts, S. A.; Hall, G. B.; Mash, E. A.; Raghunand, N. Synthetic Communic. 2014, 44, 441-449. "Improved Synthesis of 10-(2-Alkylamino-2-oxoethyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triacetic Acid Derivatives Bearing Acid-sensitive Linkers."
Jagadish, B.; Field, J. A.; Chorover, J.; Sierra-Alvarez, R.; Abrell, L.; Mash, E. A. J. Label. Compd. Radiopharm. 2014, 57, 434-436. "Synthesis of 13C and 15N Labeled 2,4-Dinitroanisole."
Mash, E. A. CrystEngComm 2014, 16, 8620-8637. "Crystal Engineering with 1,4-Piperazine-2,5-diones."
Alleti, R.; Vagner, J.; Dehigaspitiya, D. C.; Moberg, V. E.; Elshan, N. G. R. D.; Tafreshi, N. K.; Brabez, N.; Weber, C. S.; Lynch, R. M.; Hruby, V. J.; Gillies, R. J.; Morse, D. L.; Mash, E. A. Bioorg. Med. Chem. 2013, 21, 5029-5038. "Synthesis and Characterization of Time-resolved Fluorescence Probes for Evaluation of Competitive Binding to Melanocortin Receptors."
Martinez, G. V.; Navath, S.; Sewda, K.; Rao, V.; Foroutan, P.; Alleti, R.; Moberg, V. E.; Ahad, A. M.; Coppola, D.; Lloyd, M. C.; Gillies, R. J.; Morse, D. L.; Mash, E. A. Bioorg. Med. Chem. Lett. 2013, 23, 2061-2064. "Demonstration of a Sucrose-derived Contrast Agent for Magnetic Resonance Imaging of the GI Tract."
Navath, S.; Rao, V.; Woodford, R. T.; Midura-Kiela, M. T.; Ahad, A. M.; Alleti, R.; Kiela, P. R.; Mash, E. A. ACS Med. Chem. Lett. 2012, 3, 710-714. "Design, Synthesis, and Testing of a Molecular Truck for Colonic Delivery of 5-Aminosalicylic Acid."
Mash, E. A. (2012) "Synthetically Derived Chiral Auxiliaries: Uses of Derivatives of Non-Carbohydrate Aldehydes and Ketones in Asymmetric Synthesis." In: Carreira E.M. and Yamamoto H. (eds.) Comprehensive Chirality, Volume 3, pp. 377-407; Amsterdam: Elsevier.
Wells, K.E.; Weatherhead, R. A.; Murigi, F. N.; Nichol, G. S.; Carducci, M. D.; Selby, H. D.; Mash, E. A. Cryst. Growth Des. 2012, 12, 5056-5068. "Organic Crystal Engineering with 1,4-Piperazine-2,5-diones. 8. Synthesis, Crystal Packing, and Thermochemistry of Piperazinediones Derived from 2-Amino-4,7-dialkoxyindan-2-carboxylic Acids."
Jagadish, B.; Guntle, G. P.; Zhao, D.; Gokhale, V.; Ozumerzifon, T. J.; Ahad, A. M.; Mash, E. A.; Raghunand, N. J. Med. Chem. 2012, 55, 10378-10386. "Redox-active Magnetic Imaging Contrast Agents: Studies with Thiol-bearing 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetracetic Acid Derivatives."






