Professor
Degrees and Appointments
- B.S. 1969, Indiana University, Bloomington
- Ph.D. 1974, University of Wisconsin, Madison
- Postdoctoral 1974-1976, University of Illinois, Urbana-Champaign
Field of Study: Physical Chemistry, Inorganic Chemistry
Awards and Honors
- Fellow of the American Chemical Society (ACS), elected 2016
- Fellow of the American Association for the Advancement of Science (AAAS), elected 2015
- Fellow of the Galileo Circle, College of Science, elected 2012
- Associate Editor, Organometallics, 2008-2014
- Chair-elect and Chair, Organometallic Subdivision of the Inorganic Division of the ACS, 2010-2011
- Ninth Annual University Graduate and Professional Education Teaching and Mentoring Award, 2008
- Coates Lectureship, University of Wyoming, 2006
- Plenary Lecturer, Taller de Quimica Cinvestav, Mexico City, 2004
- Faculty of Science Distinguished Teaching Award, 1994
Research Specialties: Chemical Reaction Dynamics/Kinetics/Interactions, Energy Science, Instrument Development, Spectroscopy/Molecular Structure, Surface and Solid State, Synthesis/Synthetic Methods Development, Theory, Modeling, and Simulation
For more information please visit the Lichtenberger Group Website.
See the Google Scholar Profile of Professor Lichtenberger.
Research
This research program is exploring and developing new areas of chemical reactivity and catalysis by controlling the movement of electrons. An obvious example is electrocatalysis, which is a central theme of our studies, but in a broader sense all chemical behavior may be viewed as the movement of electrons. This includes the oxidation and reduction processes of metalloenzymes in biology, the selective making and breaking of bonds in industrial catalysis, the transport of electrons in molecular wires, and the interactions of molecules with light. Chemical reactivity and catalysis depend on mechanisms that move electrons from existing bonds in starting materials to new bonds in desired products. Movement of electrons in materials is important to electrical conductivity and optical properties for technological applications. In biology, electron transfer involving active metal sites is central to many life processes. This research provides fundamental information for understanding all of these processes - the clues for solving the many mysteries of electron behavior in chemistry.
ELECTROCATALYSTS FOR HYDROGEN PRODUCTION

HYDROGEN RESEARCH GROUP STUDENTS, JANUARY, 2020
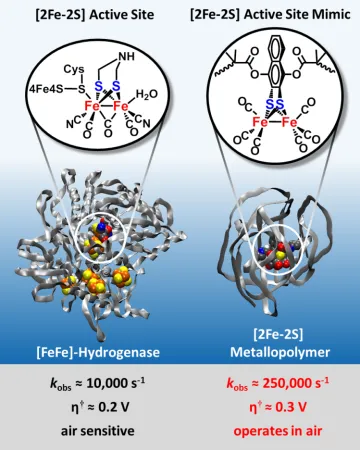
Recently we have made tremendous (and somewhat serendipitous) discoveries in the production of hydrogen by electrocatalysis. Research is underway to gain a better understanding of the key features that are responsible for the remarkable catalytic performance that we have observed. This insight is leading to new pathways to improved catalysis that can have broad and important impacts. The development of clean and sustainable sources of energy and fuels is a grand challenge for society, and electrocatalytic production of hydrogen (H2) is widely recognized to be an attractive approach to store the energy from intermittent sources such as solar and wind. Electrochemical water splitting to generate H2 and O2 typically uses expensive and rare platinum metal. Our approach is inspired by nature, where multiple-electron reductions are achieved in enzymes with multiple-metal catalytic active sites composed of earth-abundant elements, such as the [2Fe-2S] cluster organometallic sites of hydrogenases, which catalyze the reduction of protons to H2 with high rates, low energy input, and with inexpensive iron and sulfur. We are synthesizing and characterizing the catalytic activity of a wide range of [2Fe-2S] cluster catalysts. Our goals are to (1) increase the activity and chemical stability of the catalysts, (2) lower their energy requirement, (3) use water as the solvent, (4) inhibit aggregation while maintaining rapid electron transfer to the active site, and (5) increase aerobic stability. In our research we are incorporating these [2Fe-2S] active sites into polymers that afford advances in all of the outlined challenges and provide high-performance catalysts for the hydrogen evolution reaction (HER) in neutral water, as depicted in the following diagram.
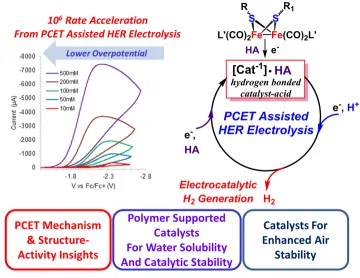
We have discovered a highly scalable new synthetic method to a metallopolymer homogeneous catalyst system for hydrogen production from water which (a) gives quantitative Faradaic yield of hydrogen, (b) is profoundly faster than [FeFe]-hydrogenases, (c) achieves current densities of more than 100 mA/cm2 at extremely low (2 ppm) catalyst loadings, (d) yields a turnover number greater than 105 molecules of hydrogen per metallopolymer catalyst site and (e) operates aerobically. The figures of merit for this catalyst from the combined metrics of low overpotential requirement for high current density (overpotential <0.2 V relative to platinum in the current density range 1 to 200 mA/cm2) substantially exceeds that of any other reported hydrogenase mimic catalysts for electrocatalytic HER and approaches the realm of heterogeneous catalysts as exemplified by platinum. Furthermore, this system offers many opportunities to tune and optimize the catalytic behavior, such as modifications of the polymer chain including utilization of other vinylic monomers, and modification of the active catalyst site for example with substitution of the dithiolate or carbonyl ligands to further lower the overpotential and increase the rate and turnover number.
SUPER-ELECTRON-DONORS.
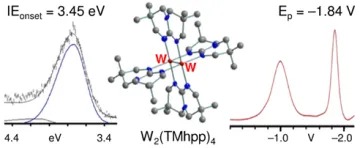
Another area of focus has been strong neutral electron donors, termed "super-electron-donors". These are characterized by low ionization energies and very negative oxidation potentials (E1/2 values beyond –1 V vs. SCE). Among the advantages for these electron donors is the ability to (1) modulate their redox potentials and reactivity by structural modifications, (2) tune their selectivity, (3) use them as pure compounds in appropriate quantities, (4) operate under mild conditions, (5) carry out reactions in organic solvents, (6) attach them to solid supports, and (7) regenerate them after use. The inherently most powerful neutral molecule electron donors yet known, as evidenced by our photoelectron measures of the extremely low gas-phase ionization energies and our electrochemical measures of extremely negative oxidation potentials, are the dimetal tetraguanidinate complexes of type shown in the Figure below.
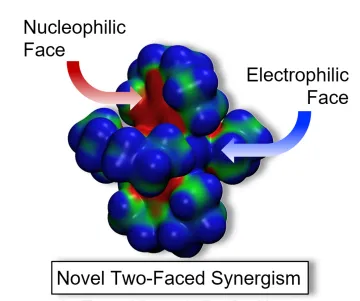
Recently we have discovered that in addition to the strong electron donor ability of these complexes, they also possess a unique bifunctionality for catalysis. This is illustrated in the electrostatic potential map shown below. Areas of high electron density potential are shown in red and areas of positive potential are shown in blue. High electron density is found throughout the ligand core and into the delta-lobes of the quadruple metal–metal bond. These sites can be thought of as nucleophilic. Protons and hydrogen bonding are drawn to these nucleophilic sites. In contrast, the M–M axial sites are electrophilic, attracting electron pairs and anions. Hence these complexes are two-faced bifunctional, with a nucleophilic face in the ligand cores and an electrophilic face at the M–M axial sites. These sites are constrained from each other by the rigidity of the structure, and thereby constitute the two components of frustrated Lewis pairs (FLPs). FLPs are known to have a rich chemistry. We are just beginning to explore the novel chemistry of the FLPs of these complexes.
Opportunities. The research offers opportunities in synthesis of new molecules and materials, electrochemistry, high-resolution gas-phase spectroscopy, ultra-high vacuum surface analysis, computational chemistry, and/or development of new methods and instrumentation. Students choose the projects in which they are most interested, and may focus on inorganic, physical, analytical, organic, or biological chemistry areas.
-
100th Anniversary of Macromolecular Science Viewpoint: High Refractive Index Polymers from Elemental Sulfur for Infrared Thermal Imaging and Optics,Tristan S. Kleine, Richard S. Glass, Dennis L. Lichtenberger, Michael E. Mackay, Kookheon Char, Robert A. Norwood, Jeffrey Pyun, ACS Macro Lett., 2020, 9, 245-259. https://dx.doi.org/10.1021/acsmacrolett.9b00948
-
Synthesis of Metallopolymers via Atom Transfer Radical Polymerization from a [2Fe‐2S] Metalloinitiator: Molecular Weight Effects on Electrocatalytic Hydrogen Production, Metin Karayilan, Keelee Cathleen McCleary‐Petersen, Meghan O'Brien Hamilton, Liye Fu, Krzysztof Matyjaszewski, Richard S. Glass, Dennis L. Lichtenberger, Jeffrey Pyun, Macromol. Rapid Commun., 2019, 1900424-30. https://dx.doi.org/10.1002/marc.201900424
-
Infrared Fingerprint Engineering: A Molecular‐Design Approach to Long‐Wave Infrared Transparency with Polymeric Materials, Tristan S. Kleine, Taeheon Lee, Kyle J. Carothers, Meghan O. Hamilton, Laura E. Anderson, Liliana Ruiz Diaz, Nicholas P. Lyons, Keith R. Coasey, Wallace O. Parker Jr., Ludovico Borghi, Michael E. Mackay, Kookheon Char, Richard S. Glass, Dennis L. Lichtenberger, Robert A. Norwood, Jeffrey Pyun, Angew. Chem. Int. Ed., 2019, 58, 17656-17660. https://dx.doi.org/10.1002/anie.201910856
-
Water-soluble and Air-stable [2Fe-2S]-Metallopolymers: A New Class of Electrocatalysts for H2 Production via Water Splitting, Richard S. Glass, Jeffrey Pyun, Dennis L. Lichtenberger, William P. Brezinski, Metin Karayilan, Kayla E. Clary, Nicholas G. Pavlopoulos, Krzysztof Matyjasezewski, Sipei Li, Liye Fu, and Dennis H. Evans, Phosphorus, Sulfur and Silicon and the Related Elements, 2019, 194(7), 701-706. https://dx.doi.org/10.1080/10426507.2019.1603705
-
Catalytic Metallopolymers from [2Fe-2S] Clusters: Artificial Metalloenzymes for Hydrogen Production, , Metin Karayilan, William P. Brezinski, Kayla E. Clary, Dennis L. Lichtenberger,* Richard S. Glass,* and Jeffrey Pyun, Angewandte Chemie (International ed.), 2019, 58(23), 7537-7550. (http://dx.doi.org/10.1002/anie.201813776
-
Weak Acids with Super-Electron-Donor Dimetal Complexes: Synergy in Bifunctional Activity, Matthew E. Humphries, Emily S. Wusterbarth, Dennis L. Lichtenberger, Polyhedron, 2019, 158, 471-477. (http://dx.doi.org/10.1016/j.poly.2018.11.001
-
Macromolecular Engineering of the Outer Coordination Sphere of [2Fe-2S] Metallopolymers to Enhance Catalytic Activity for H2 Production, William P. Brezinski, Metin Karayilan, Kayla E. Clary, Keelee C. McCleary-Petersen, Liye Fu, Krzysztof Matyjaszewski, Dennis H. Evans, Dennis L. Lichtenberger, Richard S. Glass, and Jeffrey Pyun, ACS Macro Letters, 2018, 7(11), 1383-1387. (http://dx.doi.org/10.1021/acsmacrolett.8b00765
-
[FeFe]‐Hydrogenase Mimetic Metallopolymers with Enhanced Catalytic Activity for Hydrogen Production in Water, William P. Brezinski, Metin Karayilan, Kayla E. Clary, Nicholas G. Pavlopoulos, Sipei Li, Liye Fu, Krzysztof Matyjaszewski, Dennis H. Evans, Richard S. Glass, Dennis L. Lichtenberger, Jeffrey Pyun, Angewandte Chemie (International ed.), 2018, 57(37), 11898-11902. (http://dx.doi.org/10.1002/anie.201804661
-
Vibrational Control of Covalency Effects Related to the Active Sites of Molybdenum Enzymes, Benjamin W. Stein, Jing Yang, Regina Mtei, Nicholas J. Wiebelhaus, Dominic K. Kersi, Jesse LePluart, Dennis L. Lichtenberger, John H. Enemark*, Martin L. Kirk, Journal of the American Chemical Society, 2018, 140(44), 14777-14788. (http://dx.doi.org/10.1021/jacs.8b08254
-
[FeFe]-Hydrogenase H-Cluster Mimics with Unique Planar μ-(SCH2 )2 ER2 Linkers (E=Ge and Sn), Hassan Abul‐Futouh, Laith R. Almazahreh, Takahiro Sakamoto, Nhu Y. T. Stessman, Dennis L. Lichtenberger, Richard S. Glass, Helmar Görls, Mohammad El‐Khateeb, Philippe Schollhammer, Grzegorz Mloston, Wolfgang Weigand, Chemistry: a European Journal, 2017, 23(2), 346-359. (http://dx.doi.org/10.1002/chem.201603843
-
Experimental measure of metal-alkynyl electronic structure interactions by photoelectron spectroscopy: (η5-C5H5)Ru(CO)2CCMe and (η5-C5H5)Ru(CO)2]2(μ-CC), Ashley R. Head, Sharon K. Renshaw, Andrew B. Uplinger, Jeffrey R. Lomprey, John P. Selegue and Dennis L. Lichtenberger. Polyhedron, 2015, 86, 141-150. (http://dx.doi.org/10.1016/j.poly.2014.07.020)
-
Through-space interaction mediated by a sulfoxide, G. J. Meyer, Elliott R. Smith, Takahiro Sakamoto, Dennis L. Lichtenberger and Richard S. Glass. Phosphorus Sulfur, 2015, 190, 1242-1246. (http://dx.doi.org/10.1080/10426507.2014.991822)
-
Through space interaction between ferrocenes mediated by a thioether, G. J. Meyer, Gabriel B. Hall, Elliott R. Smith, Takahiro Sakamoto, Dennis L. Lichtenberger and Richard S. Glass. Polyhedron, 2015, 86, 125-132. (http://dx.doi.org/10.1016/j.poly.2014.06.050)
-
From gas-phase ionization energies to solution oxidation potentials: Dimolybdenum tetraformamidinate paddlewheel complexes, Laura O. Van Dorn, Susan C. Borowski and Dennis L. Lichtenberger. Inorg. Chim. Acta, 2015, 424, 316-321. (http://dx.doi.org/10.1016/j.ica.2014.09.021)
-
Phosphine-substituted (η5-pentadienyl) manganese carbonyl complexes: Geometric structures, electronic structures, and energetic properties of the associative substitution mechanism, including isolation of the slipped η3-pentadienyl associative intermediate, de la Cruz Cruz, Jose Ignacio, Patricia Juarez-Saavedra, Brenda Paz-Michel, Marco Leyva-Ramirez, Asha Rajapakshe, Aaron K. Vannucci, Dennis L. Lichtenberger and M. Paz-Sandoval. Organometallics, 2014, 33, 278-288. (http://dx.doi.org/10.1021/om401017t)
-
The 2014 organometallics symposium, John A. Gladysz, Manfred Bochmann, Deryn E. Fogg, Francois P. Gabbai, Dennis L. Lichtenberger, Lanny S. Liebeskind and Daniel J. Mindiola. Organometallics, 2014, 33, 5049-5051. (http://dx.doi.org/10.1021/om500959g)
-
Intramolecular electron transfer in bipyridinium disulfides, Gabriel B. Hall, Rudresha Kottani, Greg A. N. Felton, Takuhei Yamamoto, Dennis H. Evans, Richard S. Glass and Dennis L. Lichtenberger. J. Am. Chem. Soc., 2014, 136, 4012-4018. (http://dx.doi.org/10.1021/ja500087m)
-
Effects of alkane linker length and chalcogen character in FeFe]-hydrogenase inspired compounds, Mohammad K. Harb, Ahmad Daraosheh, Helmar Goerls, Elliott R. Smith, G. J. Meyer, Matthew T. Swenson, Takahiro Sakamoto, Richard S. Glass, Dennis L. Lichtenberger, Dennis H. Evans, Mohammad El-khateeb and Wolfgang Weigand. Heteroat. Chem., 2014, 25, 592-606. (http://dx.doi.org/10.1002/hc.21216)
-
Electrochemical, spectroscopic, and computational study of bis(mu-methylthiolato)diironhexacarbonyl: Homoassociative stabilization of the dianion and a chemically reversible reduction/reoxidation cycle, Orrasa In-noi, Kenneth J. Haller, Gabriel B. Hall, William P. Brezinski, Jacob M. Marx, Taka Sakamoto, Dennis H. Evans, Richard S. Glass and Dennis L. Lichtenberger. Organometallics, 2014, 33, 5009-5019. (http://dx.doi.org/10.1021/om5004122)
-
New author guidelines for 2014: A format for computational structural data that can be opened with freely available programs such as "Mercury", Dennis L. Lichtenberger and John A. Gladysz. Organometallics, 2014, 33, 835. (http://dx.doi.org/10.1021/om500109u)
-
Introduction to the special issue on organometallic electrochemistry, Jiri Ludvik, Dennis H. Evans and Dennis L. Lichtenberger. Organometallics, 2014, 33, 4513-4516. (http://dx.doi.org/10.1021/om5008709)
-
Solubilizing the most easily ionized molecules and generating powerful reducing agents, Gina M. Chiarella, F. A. Cotton, Jason C. Durivage, Dennis L. Lichtenberger and Carlos A. Murillo. J. Am. Chem. Soc., 2013, 135, 17889-17896. (http://dx.doi.org/10.1021/ja408291k)
-
Redox chemistry of non−innocent quinones annulated to 2Fe2S cores, Gabriel B. Hall, Jinzhu Chen, Charles A. Mebi, Noriko Okumura, Matthew T. Swenson, Stephanie E. Ossowski, Uzma I. Zakai, Gary S. Nichol, Dennis L. Lichtenberger, Dennis H. Evans and Richard S. Glass. Organometallics, 2013, 32, 6605-6612. (http://dx.doi.org/10.1021/om400913p)
-
The electronic states of pyridine-N-oxide studied by VUV photoabsorption and ab initio configuration interaction computations, Michael H. Palmer, Soren V. Hoffmann, Nykola C. Jones, Elliott R. Smith and Dennis L. Lichtenberger. J. Chem. Phys., 2013, 138, 214317/1-214317/10. (http://dx.doi.org/10.1063/1.4807841)
-
Synthesis and characterization of [FeFe]-hydrogenase mimics appended with a 2-phenylazopyridine ligand, Raphael A. Seidel, Gabriel B. Hall, Matthew T. Swenson, Gary S. Nichol, Dennis L. Lichtenberger, Dennis H. Evans and Richard S. Glass. J. Sulfur Chem., 2013, 34, 566-579. (http://dx.doi.org/10.1080/17415993.2013.796553)
-
{1,1'-(Dimethylsilylene)bismethanechalcogenolato]}diiron complexes [2Fe2E(Si)] (E = S, Se, Te) - FeFe] hydrogenase models, Ulf-Peter Apfel, Helmar Goerls, Greg A. N. Felton, Dennis H. Evans, Richard S. Glass, Dennis L. Lichtenberger and Wolfgang Weigand. Helv. Chim. Acta, 2012, 95, 2168-2175. (http://dx.doi.org/10.1002/hlca.201200429)
-
The inaugural 2012 organometallics symposium, John A. Gladysz, Manfred Bochmann, Francois P. Gabbai, Dennis L. Lichtenberger, Lanny S. Liebeskind and Tobin J. Marks. Organometallics, 2012, 31, 7303-7305. (http://dx.doi.org/10.1021/om300991w)
-
Substituent effects on the electronic characteristics of pentacene derivatives for organic electronic devices: dioxolane-substituted pentacene derivatives with triisopropylsilylethynyl functional groups, Olga Lobanova Griffith, John E. Anthony, Adolphus G. Jones, Ying Shu, and Dennis L. Lichtenberger. J. Am. Chem. Soc., 2012, 134, 14185-14194. (http://dx.doi.org/10.1021/ja3056672)
-
Comparison of S and Se dichalcogenolato [FeFe]-hydrogenase models with central S and Se atoms in the bridgehead chain, Mohammad K. Harb, Jochen Windhager, Tobias Niksch, Helmar Goerls, Takahiro Sakamoto, Elliott R. Smith, Richard S. Glass, Dennis L. Lichtenberger, Dennis H. Evans, Mohammad El-Khateeb and Wolfgang Weigand. Tetrahedron, 2012, 68, 10592-10599. (http://dx.doi.org/10.1016/j.tet.2012.10.021)
-
The electronic states of 1,2,4-triazoles: A study of 1H- and 1-methyl-1,2,4-triazole by vacuum ultraviolet photoabsorption and ultraviolet photoelectron spectroscopy and a comparison with ab initio configuration interaction computations, Michael H. Palmer, Philip J. Camp, Søren Vrønning Hoffmann, Nykola C. Jones, Ashley R. Head, and Dennis L. Lichtenberger. J. Chem. Phys., 2012, 136, 094310 (11 pages). (http://dx.doi.org/10.1063/1.3692164)
-
Catalysis of electrochemical reduction of weak acids to produce H2: Role of O-H...S hydrogen bonding, Jinzhu Chen, Aaron K. Vannucci, Charles A. Mebi, Noriko Okumura, Susan C. Borowski, L. T. Lockett, Matthew Swenson, Dennis L. Lichtenberger, Dennis H. Evans and Richard S. Glass. Phosphorus, Sulfur Silicon Relat. Elem., 2011, 186, 1288-1292. (http://dx.doi.org/10.1080/10426507.2010.523035)
-
Diiron dichalcogenolato (Se and Te) complexes: Models for the active site of [FeFe] hydrogenase, Mohammad K. Harb, Ulf-Peter Apfel, Takahiro Sakamoto, Mohammad El-khateeb and Wolfgang Weigand. Eur. J. Inorg. Chem., 2011, 986-993. (http://dx.doi.org/10.1002/ejic.201001112)
-
Metal-Sulfur Valence Orbital Interaction Energies in Metal–Dithiolene Complexes: Determination of Charge and Overlap Interaction Energies by Comparison of Core and Valence Ionization Energy Shifts, Nicholas J. Wiebelhaus, Matthew A. Cranswick, Eric L. Klein, L. Tori Lockett, Dennis L. Lichtenberger,* and John H. Enemark. Inorg. Chem., 2011, 50, 11021-11031. (http://dx.doi.org/10.1021/ic201566n)
-
Thermodynamics of the metal-hydrogen bonds in(η5-C5H5)M(CO)2H (M = Fe, Ru, Os), Deven P. Estes, Aaron K. Vannucci, Ariel R. Hall, Dennis L. Lichtenberger, Jack R. Norton. Organometallics, 2011, 30, 3444-3447. (http://dx.doi.org/10.1021/om2001519)
-
Assessment of the Electronic Structure of 2,2’-pyridylpyrrolides as Ligands, Jaime A. Flores, José G. Andino, Nikolay P. Tsvetkov, Maren Pink, Robert J. Wolfe, Ashley R. Head, Dennis L. Lichtenberger, Joseph Massa, and Kenneth G. Caulton. Inorg. Chem., 2011, 50, 8121-8131. (http://dx.doi.org/10.1021/ic2005503)
-
The Electronic States of 1,2,3-Triazole Studied by Vacuum UV Photoabsorption and UV Photoelectron Spectroscopy, and a Comparison with ab initio Configuration Interaction Methods, Michael H. Palmer, Søren Vrønning Hoffmann, Nykola C. Jones, Ashley R. Head, Dennis L. Lichtenberger. J. Chem. Phys., 2011, 134, 084309 (13 pages). (http://dx.doi.org/10.1063/1.3549812)
-
Catalysis of Electrochemical Reduction of Weak Acids to Produce H2: Role of O-H•••S Hydrogen Bonding, Jinzhu Chen, Aaron K. Vannucci, Charles A. Mebi, Noriko Okumura, Susan C. Borowski, L. Tori Lockett, Matthew Swenson, Dennis L. Lichtenberger, Dennis H. Evans, and Richard S. Glass. Phosphorus, Sulfur and Silicon and the Related Elements, 2010, 186, 1288-1292. Invited paper. (http://dx.doi.org/10.1080/10426507.2010.523035)
-
Dedication to the Special Volume for Dietmar Seyferth. Manfred Bochmann, Maurice Brookhart, John A. Gladysz, Dennis L. Lichtenberger, Lanny S. Liebeskind, Tobin J. Marks, Richard R. Schrock, Dwight A. Sweigart, Kenton H. Whitmire. Organometallics, 2010 29 (21), 4647-4647. (http://dx.doi.org/10.1021/om100975m)
-
Another Great Day for Organometallic Chemistry, John A. Gladysz, Manfred Bochmann, Dennis L. Lichtenberger, Lanny S. Liebeskind, Tobin J. Marks, Dwight A. Sweigart. Organometallics, 2010 29 (22), 5737-5737. (http://dx.doi.org/10.1021/om101007r)
-
Synthesis of Diiron Hydrogenase Mimics Bearing Hydroquinone and Related Ligands. Electrochemical and Computational Studies of the Mechanism of Hydrogen Production and the Role of O–H•••S Hydrogen Bonding, Jinzhu Chen, Aaron K. Vannucci, Charles A. Mebi, Noriko Okumura, Susan C. Borowski, L. Tori Lockett, Dennis L. Lichtenberger, Dennis H. Evans and Richard S. Glass. Organometallics, 2010, 29, 5330-5340.(http://dx.doi.org/10.1021/om100396j) invited paper.
-
Phosphonothioate Hydrolysis through Selective P-S Bond Scission by Molybdenum Metallocenes, Louis Y. Kuo, Curtis P. Smith, Dennis L. Lichtenberger and Ashley R. Head. Main Group Chemistry, 2010, 9, 283-295. (http://dx.doi.org/10.1021/jp103026q)
-
Synthesis and Characterization of [FeFe]-Hydrogenase Models with Bridging Moieties Containing (S, Se) and (S, Te), Mohammad K. Harb, Helmar Gorls, Taka Sakamoto, Greg A. N. Felton, Dennis H. Evans, Richard S. Glass, Dennis L. Lichtenberger, Mohammad El-khateeb, Wolfgang Weigand. Eur. J. Inorg.Chem., 2010, 25, pp 3975-3985. http://dx.doi.org/10.1002/ejic.201000278






